Solvation, rotational relaxation and fluorescence correlation spectroscopic study on ionic liquid-in-oil microemulsions containing triple-chain surface active ionic liquids (SAILs)†
Abstract
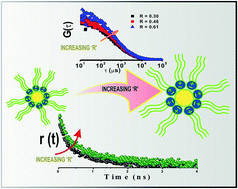
In this article, solvation dynamics and rotational relaxation approaches have been applied to explore the microheterogeneity of surface active ionic liquid (SAIL) containing microemulsions, i.e., [P13][Tf2N]/[BHD][AOT]/[IPM] and [N3111][Tf2N]/[BHD][AOT]/[IPM], using coumarin 480 (C-480) as a probe molecule. The average solvation time constants of Coumarin-480 (C-480) decreases with an increase in R value (R = [P13][Tf2N] or [N3111][Tf2N]/[BHD][AOT]) at a temperature of 25 °C for both the systems. This is attributed to the movement of the probe molecules from the interface to the pool of the microemulsion. However, the rotational relaxation time of the probe molecules (C-480) increases with increasing value of R as the pool of the microemulsion is more viscous compared to the interfacial region. Besides these, for the first time, we have measured the diffusion coefficients of these novel aggregates applying fluorescence correlation spectroscopic technique. The decrease in diffusion coefficients (Dt) with increase in R confirms the swelling of the microemulsions with addition of RTILs.

 Please wait while we load your content...
Please wait while we load your content...