RedOx-controlled sorption of iodine anions by hydrotalcite composites†
Abstract
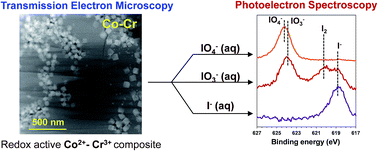
The radioactive contaminant iodine-129 (I-129) is one of the top risk drivers at radiological waste disposal and contaminated groundwater sites where nuclear material fabrication or reprocessing has occurred. However, currently there are very few options available to treat I-129 in the groundwater, partially related to the complex biogeochemical behavior of iodine in the subsurface and occurrence of I-129 in the multiple chemical forms. We hypothesize that layered hydrotalcite materials containing redox active transition metal ions offer a potential solution, benefiting from the simultaneous adsorption of iodate (IO3−), and iodide (I−) anions, which exhibit different electronic and structural properties and therefore may require dissimilar hosts. To test this hypothesis, Cr3+-based materials were selected based on the rationale that Cr3+ readily reduces IO3− in solution. It was combined with either redox-active Co2+ or redox-inactive Ni2+ so that two model materials were prepared by hydrothermal synthesis including Co2+–Cr3+ or Ni2+–Cr3+ (M–Cr). Obtained M–Cr materials composed of Co2+–Cr3+ or Ni2+–Cr3+ layered hydrotalcite and small fractions of Co3O4 spinel or Ni(OH)2 theophrastite phases were structurally characterized before and after uptake of periodate (IO4−), IO3−, and I− anions. It was found that the IO3− uptake is driven by its chemical reduction to I2 and I−. Interestingly, in the Co2+–Cr3+ hydrotalcite, Co2+ and not Cr3+ serves as a reductant while in the Ni2+–Cr3+ hydrotalcite Cr3+ is responsible for the reduction of IO3−. A different uptake mechanism was identified for the IO4− anion. The Co2+–Cr3+ hydrotalcite phase efficiently uptakes IO4− by a diffusion-limited ion exchange mechanism and is not accompanied by the redox process, while Cr3+ in the Ni2+–Cr3+ hydrotalcite reduces IO4− to IO3−, I2 and I−. Iodide exhibited high affinity only to the Co–Cr material. The Co–Cr material performed remarkably well for the removal of IO3−, I− and total iodine from the groundwater collected from the US DOE Hanford site, WA, USA, outperforming non-redox active hydrotalcites (e.g., Mg2+–Al3+) reported previously. This work demonstrates that redox-controlled sorption can be a highly effective method for the treatment of anions based on elements with mobile oxidation states. Further, multiple anions of interest could be simultaneously removed through a combination of approaches.

 Please wait while we load your content...
Please wait while we load your content...