Electrically bistable Ag nanocrystal-embedded metal–organic framework microneedles†
Keum Hwan Park‡
a,
Mun Ho Kim‡b,
Sang Hyuk Im*c and
O Ok Park*d
aDisplay Materials & Components Research Center, Korea Electronics Technology Institute, 25 Saenari-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, 13509, Republic of Korea
bDepartment of Polymer Engineering, Pukyong National University, 365 Sinseon-ro, Nam-gu, Busan 64547, Republic of Korea
cFunctional Crystallization Center (ERC), Department of Chemical Engineering, Kyung Hee University, 1732 Deogyeong-daero, Giheung-gu, Yongin-si, Gyeonggi-do 446-701, Republic of Korea. E-mail: imromy@khu.ac.kr
dDepartment of Chemical and Biomolecular Engineering (BK21+ Graduate Program), Korea Advanced Institute of Science and Technology, 291 Daehak-ro, Yuseong gu, Daejeon 305-701, Republic of Korea. E-mail: oopark@kaist.ac.kr
First published on 4th July 2016
Abstract
Ag nanocrystal-embedded metal–organic frameworks (MOF) were synthesized using a one-pot synthetic method by introducing melamine into a polyol process for the synthesis of Ag nanocrystals. The resulting MOF frameworks showed needle-like structures, inside which many Ag nanocrystals smaller than 10 nm were uniformly embedded, and exhibited electrical bistability reproducibly.
Metal nanocrystal-embedded organic/inorganic materials have been of great interest because of their potential in applications such as electrically bistable nonvolatile memory devices, microelectronics, gas adsorption, sensors, catalysts, photocatalysts, drug delivery, and biomedical applications.1–10 In particular, the electrical properties of an organic thin film can be dramatically modified by embedding the metal nanocrystals within the film because the embedded metal nanocrystals themselves can store charges.11–14 Generally, the embedding of metal nanocrystals within organic materials has been demonstrated by simply blending the nanocrystals and organic matrix materials. For instance, Ouyang et al. demonstrated the production of electrically bistable memory devices by blending synthesized Au nanocrystals with organic materials.15 Tseng et al. made a nonvolatile memory device by using polyaniline nanofibers embedded with Au nanocrystals.16
A uniform dispersion of metal nanocrystals embedded in an organic/inorganic material appears to be very important for reproducibly achieving a consistent performance. We therefore expected metal–organic framework (MOF) to be one of good candidates for use in electrically bistable devices because MOF already have periodically repeating metal-binding sites, and can hence promote site selective growth of metal nanocrystals within their frameworks. MOFs can be considered to be coordination polymers, which consist of metal ions coordinated to organic molecules. A facile synthetic strategy for the crystal engineering of coordination polymers involves the self-assembly of metal ions and organic ligands; thus, the selection of an organic ligand whose shape, coordination sites, flexibility, and symmetry are all suitable is of critical importance.17
Here, we used melamine and AgNO3 as a model organic molecule and metal ion, respectively, because it is well known that melamine forms good coordination complexes with Ag cation (Ag(I)) due to its rigid triazine ring with three reactive amino groups.18 In fact, more generally, melamine has been recognized as an ideal organic ligand for the formation of complex metal–organic structures, and so far various metal–melamine frameworks have been synthesized.19–21 However, such metal–melamine frameworks cannot exhibit electrical bistability because the metal ions are coordinated to the melamine organic matrix in such a way that charges cannot be stored in the metal ion sites. Therefore, nanocrystals of the elemental metal should be embedded in the metal–melamine frameworks. To embed such nanocrystals in metal–melamine frameworks, we here adapted the polyol process to synthesize Ag nanocrystals, and by applying the above considerations we were able to synthesize Ag nanocrystal-embedded MOF microneedles that displayed electrical bistability.
Fig. 1 shows a schematic illustration of how we produced the Ag nanocrystal-embedded MOF and what they probably look like on the atomic level. In a typical synthesis, 3.0 mL of a 94 mM AgNO3/1,5-pentanediol (PD) solution, 3.0 mL of a 147 mM polyvinylpyrrolidone (PVP: Mw = 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, in terms of PVP repeating unit)/PD solution, 1.5 mL of a 10.3 mM melamine/PD solution, and 4.5 mL of PD solvent were mixed in a vial and heated at 160 °C with magnetic stirring. Note also that we used excess Ag(I) precursor (282 μmol) relative to the melamine organic ligand (15.45 μmol) in order to in situ grow the Ag nanocrystals in Ag(I)–melamine MOF via the polyol process. During the reaction, the melamine molecules have previously been shown to generally to be bonded to Ag(I) through their ring nitrogens, forming Ag(I)–N bonds.18–21 Since the coordination number of Ag(I) can be 2, the melamine molecules can be connected to each other through Ag–N bonds to form N–Ag(I)–N chains and consequently promote the formation of one dimensional morphologies such as wires, bars, and needles. Two of the three ring nitrogens of each melamine molecule would be expected to be bonded to Ag(I) within an Ag(I)–melamine chain, and the remaining ring nitrogen atom can be involved in hydrogen bonding with the adjacent Ag(I)–melamine chain. Therefore, the linear Ag(I)–melamine complexes can be held together by inter-chain hydrogen bonds between the melamine molecules, giving rise to an anisotropic nanostructure with a high aspect ratio.18–21 Simultaneously, the excess Ag(I) ions would be expected to be reduced to Ag atoms by PD polyol so that nanocrystals of the reduced Ag atoms might grow within Ag–melamine MOF due to the affinity between the Ag(I) in the MOF and the reduced Ag atoms as illustrated in Fig. 1.
000, in terms of PVP repeating unit)/PD solution, 1.5 mL of a 10.3 mM melamine/PD solution, and 4.5 mL of PD solvent were mixed in a vial and heated at 160 °C with magnetic stirring. Note also that we used excess Ag(I) precursor (282 μmol) relative to the melamine organic ligand (15.45 μmol) in order to in situ grow the Ag nanocrystals in Ag(I)–melamine MOF via the polyol process. During the reaction, the melamine molecules have previously been shown to generally to be bonded to Ag(I) through their ring nitrogens, forming Ag(I)–N bonds.18–21 Since the coordination number of Ag(I) can be 2, the melamine molecules can be connected to each other through Ag–N bonds to form N–Ag(I)–N chains and consequently promote the formation of one dimensional morphologies such as wires, bars, and needles. Two of the three ring nitrogens of each melamine molecule would be expected to be bonded to Ag(I) within an Ag(I)–melamine chain, and the remaining ring nitrogen atom can be involved in hydrogen bonding with the adjacent Ag(I)–melamine chain. Therefore, the linear Ag(I)–melamine complexes can be held together by inter-chain hydrogen bonds between the melamine molecules, giving rise to an anisotropic nanostructure with a high aspect ratio.18–21 Simultaneously, the excess Ag(I) ions would be expected to be reduced to Ag atoms by PD polyol so that nanocrystals of the reduced Ag atoms might grow within Ag–melamine MOF due to the affinity between the Ag(I) in the MOF and the reduced Ag atoms as illustrated in Fig. 1.
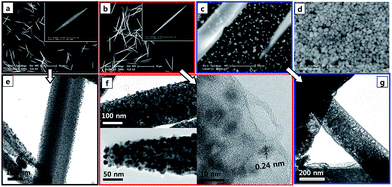
Fig. 2a–d shows scanning electron microscopy (SEM) images of the expected Ag nanocrystal-embedded Ag(I)–melamine MOF product of our reaction at various reaction times, from 5–40 min. To monitor the change in the morphology and structure of the product, we conducted several identical reactions and quenched each one at the different time points by promptly cooling down the reaction vessel (50 mL vial) in an ice bath. Since the transparent reaction solution became milky white within 5 min, we chose this time period as the first one to be tested. At this time point, needle-shaped micro-particles with an average diameter of ∼360 nm and length of ∼5 μm (aspect ratio ∼14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. 2a) were observed. A magnified view of a single microneedle appeared to show its having smooth surfaces (Fig. 2a, inset), but an even more magnified SEM image indicated that the microneedles surface to be a bit rough and covered by very tiny particles (Fig. S1a†). These tiny particles were estimated to have dimensions of about 5 nm form corresponding transmission electron microscopy (TEM) images (Fig. 2e). As the reaction time was increased to 15 min, the milky white solution became darker. The produced microneedles at this reaction time were ∼11 μm in length, with an ∼24
1) (Fig. 2a) were observed. A magnified view of a single microneedle appeared to show its having smooth surfaces (Fig. 2a, inset), but an even more magnified SEM image indicated that the microneedles surface to be a bit rough and covered by very tiny particles (Fig. S1a†). These tiny particles were estimated to have dimensions of about 5 nm form corresponding transmission electron microscopy (TEM) images (Fig. 2e). As the reaction time was increased to 15 min, the milky white solution became darker. The produced microneedles at this reaction time were ∼11 μm in length, with an ∼24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aspect ratio (Fig. 2b). A magnified SEM image of one of these microneedle also showed a smooth surface (Fig. 2b, inset), and again corresponding TEM images showed the surfaces covered with small nanoparticles, but here more densely covered than at 5 min reaction time and with the small nanoparticles <10 nm in size and also formed inside the microneedles (Fig. 2f). Moreover, these nanoparticles appeared crystalline, with a lattice distance ∼0.24 nm, which is the same as that of (111) plane of Ag crystals. These observations confirmed that Ag nanocrystals were embedded in microneedles. After a reaction time of 30 min, the reaction solution became dark yellow, which is the typical color of Ag nanocrystals. The SEM images here indicated that, at this time, the structure of the microneedle embedded with Ag nanocrystals was starting to deteriorate and many of the produced Ag nanocrystals were detached from the mother microneedles (Fig. 2c and S1b†). Moreover, the corresponding TEM image revealed a porous rather than solid microneedle structure at this time (Fig. 2g). This deterioration may have resulted from the break of N–Ag(I)–N bonds of melamine and Ag(I) in the microneedles and the reduction of the Ag+ ions constructing the MOF by the continued polyol reaction. At 40 min of reaction, no microneedles were observed, and only irregular Ag nanocrystals remained in the solution (Fig. 2d).
1 aspect ratio (Fig. 2b). A magnified SEM image of one of these microneedle also showed a smooth surface (Fig. 2b, inset), and again corresponding TEM images showed the surfaces covered with small nanoparticles, but here more densely covered than at 5 min reaction time and with the small nanoparticles <10 nm in size and also formed inside the microneedles (Fig. 2f). Moreover, these nanoparticles appeared crystalline, with a lattice distance ∼0.24 nm, which is the same as that of (111) plane of Ag crystals. These observations confirmed that Ag nanocrystals were embedded in microneedles. After a reaction time of 30 min, the reaction solution became dark yellow, which is the typical color of Ag nanocrystals. The SEM images here indicated that, at this time, the structure of the microneedle embedded with Ag nanocrystals was starting to deteriorate and many of the produced Ag nanocrystals were detached from the mother microneedles (Fig. 2c and S1b†). Moreover, the corresponding TEM image revealed a porous rather than solid microneedle structure at this time (Fig. 2g). This deterioration may have resulted from the break of N–Ag(I)–N bonds of melamine and Ag(I) in the microneedles and the reduction of the Ag+ ions constructing the MOF by the continued polyol reaction. At 40 min of reaction, no microneedles were observed, and only irregular Ag nanocrystals remained in the solution (Fig. 2d).
To confirm the expected Ag nanocrystal-embedded MOF structure of the microneedle product, we carried out Fourier transform infrared (FTIR) spectroscopy and elemental analysis (EA), as shown in Fig. 3a and Table 1. FTIR spectra of pure melamine ((a) in Fig. 3a and S2†) and the product of our reaction at reaction times of 5 min ((b) in Fig. 3a and S2†) and 15 min ((c) in Fig. 3a and S2†) showed that the semicircle stretching frequencies of the melamine triazine ring were shifted from 1464 cm−1 and 1433 cm−1 for pure melamine to 1473 cm−1 and 1446 cm−1 for the reaction product. These shifts can be attributed to the donation of a lone pair of electrons from the triazine nitrogen to Ag upon formation of the N–Ag bond.21 This result indicates that an MOF formed. The result of EA of the Ag nanocrystal-embedded MOF microneedle product after 5 min reaction is summarized in Table 1. The empirical formula of the product was determined from the EA to be C3H6.67N7.34O3.48Ag3.11, which is similar to that of the supramolecular complex C3N6H6(AgNO3).18 The greatest difference was the relative amount of Ag, indicative of the excess Ag forming nanocrystals on/within the MOF microneedles. Powder X-ray diffraction (PXRD) patterns recorded from the samples obtained after 5 min and 15 min reaction are shown in Fig. S3,† which indicated that the MOF microneedles are crystalline. The positions and relative intensities of the peaks are retained after the growth of Ag nanocrystals. In the spectrum of the sample obtained after 15 min reaction, a peak at 38.2° appeared, which corresponds to the {111} plane of Ag. Although the intensity of Ag nanocrystal in MOF microneedles seems to be weak due to the relatively smaller quantity of Ag nanocrystals than the MOF, it clearly confirmed that the frame work is maintained during the formation of Ag nanocrystals in the MOF microneedles.22
| N | C | H | O | Ag | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wt [%] | At ratio | Wt [%] | At ratio | Wt [%] | At ratio | Wt [%] | At ratio | Wt [%] | At ratio | |
| t = 5 [min] | 19.15 | 7.34 | 6.71 | 3.00 | 1.26 | 6.67 | 10.37 | 3.48 | 62.51 | 3.11 |
To gain an understanding of the reaction mechanism that formed the MOF microneedles embedded with Ag nanocrystals and then caused these microneedles to disappear, we checked the pH of the reaction solution at various reaction times, as shown in Fig. 3b. At the early stages of the reactions, the pH slightly decreased because the diols of the PD solvent oxidized, through aldehyde intermediate, to carboxylic acids. After 36 min of the reaction, however, the pH of the reaction solution abruptly increased. In contrast, in a control reaction with the same reactants but without melamine, the pH remained unchanged (data not shown). The abrupt increase in the pH of the reactant solution containing melamine might have resulted from the breaking of the coordination bonds between melamine and Ag(I) ions: most of the melamine molecules could not have acted as a base at the early stages of the reaction because their amines were bonded to Ag or to other melamines in the MOF; however, they could have begun to act as a base when the MOFs broke apart. At 40 min of reaction, the pH of the solution exceeded 7.0. At this time, the MOF microneedles were not found in the solution, indicating that most of the melamine molecules became re-dissolved into the solution. From the above experiments, we can conclude that melamine and Ag(I) combined to form the MOF microneedles embedded with Ag nanocrystals early on in the polyol synthesis, the Ag nanocrystals became more densely packed on/within MOF microneedles at intermediate reaction time, and finally, at long reaction time, the Ag nanocrystal-embedded MOF microneedles disassembled into Ag nanocrystals and melamine. However, we were able to reproducibly fabricate the Ag nanocrystals-embedded MOF microneedles by quenching the reaction within 30 min.
To find applications of the MOF microneedles embedded with Ag nanocrystals, we set out to measure their electrical bistability. To carry out this experiment, we used a single Ag nanocrystal-embedded MOF microneedle (t = 15 min sample) whose two ends were attached to Pt electrodes by using the focused ion beam (FIB) technique, as shown in Fig. 4a and b. The current–voltage (I–V) curves of this microneedle, aimed at showing its electrical bistability properties, are shown in Fig. 4c. Initially, we applied a forward voltage to the microneedle from 0 V to 3 V for the writing state. The current (I) abruptly increased at an applied forward voltage of ∼1.7 V due to the decreased resistivity of the microneedle, indicating that the OFF state of the microneedle switched to the ON state (a: write state). Then, when we scanned again the ON state of the microneedle and then measured g Ag nanocrystals from 0 V to 3 V, the current was observed to be linearly dependent on the applied forward voltage, and a higher current (b: read state) flowed below ∼1.7 V than in the initial OFF state. To delete the information, we applied a 5 V reverse voltage to the microneedle and then measured the current resulting from the applied forward voltage from 0 V to 3 V (c: rewrite state). The current was observed to abruptly increase at an applied forward voltage of ∼2 V. The I–V curve did not exactly recover to the original state, but did so reasonably well. Then we were able to read again (d: read state) as the I–V curve of this section read state matched that of the first read state (b). Although this microneedle did not show good performance, it did show reproducible electrical bistability as shown in Fig. 4d during ten sweep cycles. It should be noted that once switched from the OFF state to the ON state, the microneedle maintained the ON state without applying voltage; this observation indicates that MOF microneedles embedded with Ag nanocrystals can be used in non-volatile memory devices. Finally, we checked the electrical stability of Ag nanocrystal-embedded MOF microneedles to determine their average operating window. These microneedles broke at an applied bias voltage of over ∼50 V, as shown in Fig. S4,† which might have been caused by the decomposition of its organic material by the generated heat.
Conclusions
Ag nanocrystal-embedded MOF microneedles were synthesized in situ by introducing melamine into a polyol process, and were shown to display electrical bistability. The one-dimensional morphology of the structures could be explained by the formation of bonds between AgNO3 and melamine, leading to the formation of N–Ag(I)–N chains, and the formation of melamine–melamine hydrogen bonds between these chains. The polyol 1,5 pentanediol, which was adopted as the reaction medium, enabled the continuous formation of Ag nanocrystals in the reaction mixtures, and the formed Ag nanocrystals were embedded inside of the MOF microneedles. When we checked the electrical properties of these Ag nanocrystal-embedded MOF microneedles, they showed electrical bistability reproducibly, so that it was possible to demonstrate the write state, read state, and rewrite state. Although these microneedles did not display sufficiently high level of performance to readily apply them to memory devices, they should, after further intensive studies, find use in memory devices, sensors, photocatalysts and so on.Acknowledgements
This work was supported by the National Research Foundation of Korea (KRF) grants funded by the Ministry of Science, ICT & Future Planning (MSIP) of Korea under contact no. NRF-2015R1C1A1A02036649. This work was also partially supported by the Ministry of Trade, Industry & Energy of Korea and the Defense Acquisition Program Administration (15-CM-EN-08).Notes and references
- R. Matsuda, R. Kitaura, S. Kitagawa, Y. Kubota, R. V. Belosludov, T. C. Kobayashi, H. Sakamoto, T. Chiba, M. Takata, Y. Kawazoe and Y. Mita, Nature, 2005, 436, 238 CrossRef CAS PubMed.
- K. Na, K. M. Choi, O. M. Yaghi and G. A. Somorjai, Nano Lett., 2014, 14, 5979 CrossRef CAS PubMed.
- S. Horike, M. Dinca, K. Tamaki and J. R. Long, J. Am. Chem. Soc., 2008, 130, 5854 CrossRef CAS PubMed.
- Z. Sha and J. Wu, RSC Adv., 2015, 5, 39592 RSC.
- B. Chen, S. Xiang and G. Qian, Acc. Chem. Res., 2010, 43, 1115 CrossRef CAS PubMed.
- Z. Gu, L. Chen, B. Duan, Q. Luo, J. Liu and C. Duan, Chem. Commun., 2016, 52, 116 RSC.
- H. Xu, F. Liu, Y. Cui, B. Chen and G. Qian, Chem. Commun., 2011, 47, 3153 RSC.
- P. Falcaro, R. Ricco, A. Yazdi, I. Imaz, S. Furukawa, D. Maspoch, R. Ameloot, J. D. Evans and C. J. Doonan, Coord. Chem. Rev., 2016, 307, 237 CrossRef CAS.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. S. Chang, Y. K. Hwang, V. Marsaud, P. N. Bories, L. Cynober, S. Gil, G. Fery, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172 CrossRef CAS PubMed.
- L. Lin, T. Zhang, H. Liu, J. Qiu and X. Zhang, Nanoscale, 2015, 7, 7615 RSC.
- L. P. Ma, J. Liu and Y. Yang, Appl. Phys. Lett., 2002, 80, 2997 CrossRef CAS.
- L. P. Ma, S. Pyo, J. Ouyang, Q. Xu and Y. Yang, Appl. Phys. Lett., 2003, 82, 1419 CrossRef CAS.
- L. D. Bozano, B. W. Kean, V. R. Deline, J. R. Salem and J. C. Scott, Appl. Phys. Lett., 2004, 84, 607 CrossRef CAS.
- J. Wu, L. P. Ma and Y. Yang, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 115321 CrossRef.
- J. Ouyang, C. W. Chu, C. R. Szmanda, L. P. Ma and Y. Yang, Nat. Mater., 2004, 3, 918 CrossRef CAS PubMed.
- R. J. Tseng, J. Huang, J. Ouyang, R. B. Kaner and Y. Yang, Nano Lett., 2005, 5, 1077 CrossRef CAS PubMed.
- H. Liu, S. Ji and Y. Hao, Z. Anorg. Allg. Chem., 2014, 640, 2595 CrossRef CAS.
- K. Sivashankar, A. Ranganathan, V. R. Pedireddi and C. N. R. Rao, J. Mol. Struct., 2001, 559, 41 CrossRef CAS.
- P. Bairi, B. Roy and A. K. Nandi, J. Mater. Chem., 2011, 21, 11747 RSC.
- J. Fei, L. Gao, J. Zhao, C. Du and J. Li, Small, 2013, 9, 1021 CrossRef CAS PubMed.
- A. B. Wiles, D. Bozzuto, C. L. Cahill and R. D. Pike, Polyhedron, 2006, 25, 776 CrossRef CAS.
- Y. E. Cheon and M. P. Suh, Angew. Chem., Int. Ed., 2009, 48, 2899 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of experimental, magnified SEM images, FTIR spectra, and PXRD patterns. See DOI: 10.1039/c6ra13014c |
| ‡ These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |