Deposition of a hydrophilic nanocomposite-based coating on silicone hydrogel through a laser process to minimize UV exposure and bacterial contamination†
Abstract
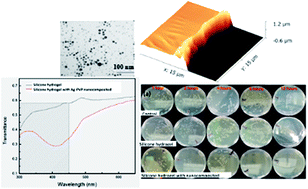
To prevent contact lens from biofouling and to minimize UV exposure to human eyes, a nanocomposite-based coating made of silver (Ag) nanoparticles and polyvinylpyrrolidone (PVP) is deposited on synthetic silicone hydrogels through matrix assisted pulsed laser evaporation (MAPLE) with a pulsed Nd:YAG laser at 532 nm. The average diameter of Ag NPs that have undergone the MAPLE process for 60 min is 11.61 ± 3.58 nm. The thickness of the Ag–PVP nanocomposite coating with a deposition time of 60 min is around 930 ± 15 nm. Our results demonstrate that the oxygen permeability of silicone hydrogel with a nanocomposite coating is similar to that of commercialized contact lenses; over 60% of UV light in the range of 300–450 nm can be blocked. Moreover, the silicone hydrogel with the nanocomposite coating can reduce over 65.4 ± 1.6% of protein (human lysozyme) absorption as compared to silicone hydrogel-based contact lens, and kill all cultured bacteria in 8 hours. This research work demonstrates a new way to deposit biocompatible nanocomposite coatings on silicone hydrogels used as contact lens to efficiently minimize UV exposure and biofouling.

 Please wait while we load your content...
Please wait while we load your content...