An extremely rapid-response fluorescent probe for hydrogen peroxide and its application in living cells†
Fuxu Zhan,
Qian Yang,
Qiufen Wang,
Qilong Zhang,
Zhiyuan Zhuang,
Xue Feng,
Guangyou Zhang and
Gengxiu Zheng*
School of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, China. E-mail: chm_zhenggx@ujn.edu.cn; Tel: +86-53182765841
First published on 8th September 2016
Abstract
A turn-on fluorescence probe ACF for rapid detection of H2O2 was constructed. The probe utilized a 2-(azidomethyl)benzoyl group as a new reaction site, which exhibited a rapid response to H2O2 with a 118-fold fluorescence enhancement within 5 min. The biological application of ACF was confirmed by fluorescence imaging H2O2 in living cells.
Hydrogen peroxide (H2O2), one of the most important reactive oxygen species (ROS), has been known as a harmful metabolic product and a component of the immune response to microbial invasion for a long time.1 However, H2O2 functions as an ubiquitous intracellular second messenger when it is generated at a low concentration (<0.7 μM).2 It stimulates cell proliferation,3 differentiation,4 and migration5 by activating the signalling pathways. H2O2 might be generated aberrantly and result in oxidative stress with stimulation by exogenous chemicals. However, H2O2 is an oxidant unlike the classical second messenger.6 The resulting H2O2 and other ROS will attack cellular structures or biomolecules such as proteins,7 liposomes,8 and DNA,9 which has been correlated with aging,10 Alzheimer's disease,11 and cancer.12 Obviously, information on the location and timing of H2O2 generation in biological processes is important and would provide information that could help to understand the function of H2O2.
Currently, several chemical methods have been developed to detect intracellular H2O2, including mass assays,13 proteomics assays,14 and fluorescence-based assays.15–21 Among these methods, fluorescence-based assays were useful because of their non-destructive features. A few fluorescent probes designed for H2O2 detection have been reported since 2003.16a The first developed and most popular probes were a kind of boronate ester,16 which have occupied more than a half of all the H2O2 probes, including NIR, ratiometric, targetable, trappable and two-photon probes etc. Some of them have been successfully applied to monitor H2O2 at physiological levels in vitro and in vivo and others have been used to explore the cellular mechanisms associated with H2O2. Chang group contributed a lot in this field.16b Another kind of fluorescent probes developed for H2O2 in the early stage contained arylsulfonyl esters as trap groups.17 After that, several kinds of probes were designed based on unique H2O2-responsive sites such as diphenylphosphine,18 α-diketone groups,19 metal complexes20 and some chalcogen.21 The efforts to find novel H2O2-responsive sites were still in progress.
Although several H2O2-responsive sites have been developed and a lot of fluorescence probes have been constructed, the reaction rates and fluorescence background levels of some fluorescence probes were generally not satisfactory for biological applications. When treated with H2O2, most of them had a long response time which became an important and complex issue for monitoring the H2O2 concentration in living cells.22 Only a few of them could respond to H2O2 fast and selectively. It is especially important to develop rapid-response probes to monitor the H2O2 in biological process.
Reduced fluorescent dyes such as 2′,7′-dichlorodihydrofluorescein diacetate were commonly used as fluorescence probes for H2O2.23 However, it still showed non-fluorescence if 2′,7′- dichlorodihydrofluorescein diacetate was only oxidized to 2′,7′-dichlorofluorescin diacetate.24 It is obvious that the ester bond was broken during or after the oxidation. In this process, H2O2 as a good nucleophile,16b might promote a nucleophilic substitution. So it is possible to construct a fluorescence probe for H2O2 based on breaking a special ester bond. Therefore, finding a special ester bond and linking it with a fluorophore may be a feasible strategy.
Based on this strategy, ACF was synthesized by the reaction of 2′,7′-dichlorofluorescein and 2-(azidomethyl)benzoyl acid (Scheme 1). ACF exhibited almost no fluorescence (fluorescence quantum yield: Φ = 0.0024, in CH3OH/PBS buffer, 10 mM, pH = 7.4, 5/95, ESI†).
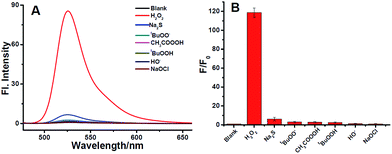
When treated with H2O2, ACF showed extremely rapid response. Only in 5 min, the fluorescent intensity was increased by 118-fold (Fig. 1). The fluorescent intensity increased quite fast in the first 4 min, after which the increase rate fell a little. After 10 min, the fluorescent intensity was almost linear to the time with a correlation coefficient of 0.9990. And when extended to 120 min, the fluorescent intensity even increased by 441-fold (fluorescence quantum yield: Φ = 0.6780, in CH3OH/PBS buffer, 10 mM, pH = 7.4, 5/95, ESI†). The fluorescent intensities in the following experiments were recorded in 5 min due to the rapid-response of ACF for H2O2. Then the effect of pH on the fluorescence of ACF was evaluated, which showed it was stable from pH 6 to 8, even in the presence of H2O2 (Fig. S1, ESI†).
To estimate the selectivity of probe ACF for H2O2, fluorescence responses to other ROS were examined. As shown in Fig. 2, significant fluorescence enhancement was observed after incubation with H2O2 for 5 min. Compared to H2O2, other ROS induced only negligible fluorescence enhancements under the same condition, including t-BuOOH, hydroxy radical, CH3CO3H, and so on. Considering 2-azidomethylbenzoate had been used as a H2S trap,25 H2S was also examined. It is true that fluorescence enhancement is observed after incubation with H2S. But the increment was far less than that of H2O2 (Fig. 2).
To further estimate the selectivity of probe ACF for H2O2, time-dependent fluorescence changes to ROS and H2S were recorded. As shown in Fig. 3, the fluorescence enhancements for other ROS were still negligible in 120 min. While the enhancement for H2S in 30 min was obvious. However, it was not strong enough to obstruct the detection of H2O2 (the fluorescent intensity for H2S to H2O2 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.6). What was more, the fluorescent intensity for H2O2 increased obviously with the time, while that for H2S almost ceased. Thus, probe ACF showed high selectivity toward H2O2.
9.6). What was more, the fluorescent intensity for H2O2 increased obviously with the time, while that for H2S almost ceased. Thus, probe ACF showed high selectivity toward H2O2.
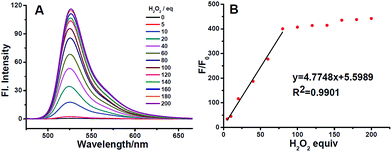
Subsequently, we examined the reactivity of ACF towards different concentrations of H2O2 in CH3OH/PBS buffer (10 mM, pH = 7.4, 5/95) at 25 °C. As expected, after incubation with H2O2, the fluorescence intensity of probe ACF increased gradually as the increase of the H2O2 amounts. Fig. 4 showed the fluorescence intensity of probe ACF increased almost linearly with the concentration of H2O2 in the range of 50–400 μM, and a correlation coefficient of 0.9901. Specifically, the detection limit of H2O2 was determined to be 6.5 nM based on the 3σ/slope method (ESI†), which was much lower than those of the reported probes. The good linearity indicated that probe ACF was able to qualitatively and quantitatively determine the level of H2O2.
Next, we carried out competition experiments in the presence of ROS and H2S (Fig. S2, ESI†). ACF was still able to respond to H2O2 with strong fluorescence enhancements in the presence of the interfering species. Moreover, the process of ACF for detection of H2O2 was confirmed by the HRMS-ESI spectra. The mass signal for 2′,7′-dichlorofluorescin ([M + H]+ calcd for C20H11Cl2O5+, 400.9978, found: 400.9962, Fig. S3, ESI†), was detected after probe ACF was incubated with H2O2. So the ester bond was possible broken by the nucleophilic substitution induced by H2O2. A controlled experiment showed that the probe would completely lose its effect if the azido group was instead by a hydrogen atom, which proved the importance of the azido group. Though the exact mechanism and the special effect of azido group were not clear, the in-depth mechanism study is in progress.
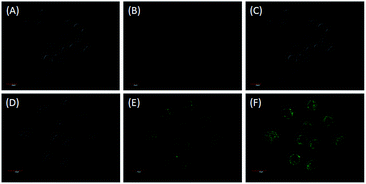
Encouraged by the above excellent results, we subsequently explored the potential applications of ACF for monitoring and imaging of H2O2 in living cells. Firstly, the cytotoxicity of ACF was evaluated using A-549 cells and Raw 264.7 cells (obtained from the College of Life Science, Nankai University, Tianjin, China; serum was purchased from Gibco) by MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Fig. S4, ESI†]. Probe ACF showed almost no cytotoxicity in 0.1–30 μM range to both of them, implying that the probe was suitable for bioimaging of H2O2 in living cells. Finally, we assessed the application of the probe for monitoring and imaging of H2O2 in living cells. HeLa cells (obtained from the College of Life Science, Nankai University, Tianjin, China) incubated with ACF (10 μM) in culture medium for 15 min at 37 °C, showed almost no fluorescence (Fig. 5B). However, if the cells were pre-treated with ACF (10 μM) for 15 min and then incubated with H2O2 (10 eq., 100 μM) for 15 min, strong fluorescence was observed (Fig. 5E). The obvious fluorescent enhancement indicated that probe ACF could image H2O2 in living cells. ACF responded to H2O2 only in 15 min, making the detection get close to real-time monitoring.
Conclusions
In conclusion, aiming at finding rapid-response fluorescent probes for detection of H2O2, a new probe 2′,7′-dichloro-3′,6′-bis(2-(azidomethyl)benzoate)fluorescein (ACF) was developed. ACF can rapidly respond to H2O2 and offer highly sensitivity and selectivity by utilizing the unique chemical reactivity of the ester bonds and H2O2. Preliminary fluorescence imaging experiments indicate that ACF is a good fluorescent tool for rapidly monitoring H2O2 in living cells. We believe the novel H2O2 response site will be broadly used for quantitatively monitoring of H2O2 in biological systems. Relevant studies on this strategy and its biological applications are underway.Acknowledgements
This work was supported by the National Science Foundation of China (21402064), the startup fund of University of Jinan, the Doctoral Fund of University of Jinan (160080304) and the Science Foundation for Post Doctorate Research from the University of Jinan (1003814).Notes and references
- J. A. Imlay, Annu. Rev. Biochem., 2008, 77, 755 CrossRef CAS PubMed.
- S. G. Rhee, S. W. Kang, W. Jeong, T. S. Chang, K. S. Yang and H. A. Woo, Curr. Opin. Cell Biol., 2005, 17, 183 CrossRef CAS PubMed.
- M. Geiszt and T. L. Leto, J. Biol. Chem., 2004, 279, 51715 CrossRef CAS PubMed.
- J. Li, M. Stouffs, L. Serrander, B. Banfi, E. Bettiol, Y. Charnay, K. Steger, K. H. Krause and M. E. Jaconi, Mol. Biol. Cell, 2006, 17, 3978 CrossRef CAS PubMed.
- M. Ushio-Fukai, Cardiovasc. Res., 2006, 71, 226 CrossRef CAS PubMed.
- B. P. Yu, Physiol. Rev., 1994, 74, 139 CAS.
- (a) E. R. Stadtman, Science, 1992, 257, 1220 CAS; (b) E. R. Stadtman, Free Radical Res., 2006, 40, 1250 CrossRef CAS PubMed.
- I. Levitan, S. Volkov and P. V. Subbaiah, Antioxid. Redox Signaling, 2010, 13, 39 CrossRef CAS PubMed.
- (a) S. Shibutani, M. Takeshita and A. P. Grollman, Nature, 1991, 349, 431 CrossRef CAS PubMed; (b) S. Kanvah, J. Joseph, G. B. Schuster, R. N. Barnett, C. L. Cleveland and U. Landman, Acc. Chem. Res., 2010, 43, 280 CrossRef CAS PubMed.
- V. A. Bohr, Free Radical Biol. Med., 2002, 32, 804 CrossRef CAS PubMed.
- C. Behl, J. B. Davis, R. Lesley and D. Schubert, Cell, 1994, 77, 817 CrossRef CAS PubMed.
- S. Loft and H. E. Poulsen, J. Mol. Med., 1996, 74, 297 CrossRef CAS PubMed.
- H. M. Cocheme, A. Logan, T. A. Prime, I. Abakumova, C. Quin, S. J. McQuaker, J. V. Patel, I. M. Fearnley, A. M. James, C. M. Porteous, R. A. Smith, R. C. Hartley, L. Partridge and M. P. Murphy, Nat. Protoc., 2012, 7, 946 CrossRef CAS PubMed.
- V. V. Belousov, A. F. Fradkov, K. A. Lukyanov, D. B. Staroverov, K. S. Shakhbazov, A. V. Terskikh and S. Lukyanov, Nat. Methods, 2006, 3, 281 CrossRef CAS PubMed.
- X. Chen, X. Tian, I. Shin and J. Yoon, Chem. Soc. Rev., 2011, 40, 4783–4804 RSC.
- (a) L.-C. Lo and C.-Y. Chu, Chem. Commun., 2003, 2728 RSC; (b) A. R. Lippert, G. C. Van de Bittner and C. J. Chang, Acc. Chem. Res., 2011, 44, 793 CrossRef CAS PubMed; (c) E. W. Miller, B. C. Dickinson and C. J. Chang, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 15681 CrossRef CAS PubMed; (d) G. C. Van de Bittner, E. A. Dubikovskaya, C. R. Bertozzi and C. J. Chang, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 21316 CrossRef CAS PubMed; (e) N. Karton-Lifshin, E. Segal, L. Omer, M. Portnoy, R. Satchi-Fainaro and D. Shabat, J. Am. Chem. Soc., 2011, 133, 10960 CrossRef CAS PubMed; (f) L. Yuan, W. Lin, Y. Xie, B. Chen and S. Zhu, J. Am. Chem. Soc., 2012, 134, 1305 CrossRef CAS PubMed; (g) G. Masanta, C. H. Heo, C. S. Lim, S. K. Bae, B. R. Cho and H. M. Kim, Chem. Commun., 2012, 48, 3518 RSC; (h) G. C. V. Bittner, C. R. Bertozzi and C. J. Chang, J. Am. Chem. Soc., 2013, 135, 1783 CrossRef PubMed; (i) E. W. Miller, O. Tulyathan, E. Y. Isacoff and C. J. Chang, Nat. Chem. Biol., 2013, 3, 263 CrossRef PubMed; (j) S. W. Lee, H.-W. Rhee, Y.-T. Chang and J.-I. Hong, Chem.–Eur. J., 2013, 19, 14791 CrossRef CAS PubMed; (k) Y. Wen, K. Liu, H. Yang, Y. Li, H. Lan, Y. Liu, X. Zhang and T. Yi, Anal. Chem., 2014, 86, 9970 CrossRef CAS PubMed; (l) W. Wu, J. Li, L. Chen, Z. Ma, W. Zhang, Z. Liu, Y. Cheng, L. Du and M. Li, Anal. Chem., 2014, 86, 9800 CrossRef CAS PubMed; (m) C. Liu, C. Shao, H. Wu, B. Guo, B. Zhu and X. Zhang, RSC Adv., 2014, 4, 16055 RSC; (n) W. Zhang, W. Liu, P. Li, F. Huang, H. Wang and B. Tang, Anal. Chem., 2015, 87, 9825 CrossRef CAS PubMed; (o) L. Yi, L. Wei, R. Wang, C. Zhang, J. Zhang, T. Tan and Z. Xi, Chem.–Eur. J., 2015, 21, 15167 CrossRef CAS PubMed.
- (a) H. Maeda, Y. Fukuyasu, S. Yoshida, M. Fukuda, K. Saeki, H. Matsuno, Y. Yamauchi, K. Yoshida, K. Hirata and K. Miyamoto, Angew. Chem., Int. Ed., 2004, 43, 2389 CrossRef CAS PubMed; (b) H. Li, Q. Li, X. Wang, K. Xu, Z. Chen, X. Gong, X. Liu, L. Tong and B. Tang, Anal. Chem., 2009, 81, 2193 CrossRef CAS PubMed; (c) K. B. Daniel, J. L. M. Jourden, K. E. Negoescu and S. M. Cohen, J. Biol. Inorg. Chem., 2011, 16, 313 CrossRef CAS PubMed.
- (a) N. Soh, O. Sakawaki, K. Makihara, Y. Odo, T. Fukaminato, T. Kawai, M. Irie and T. Imato, Bioorg. Med. Chem., 2005, 13, 1131 CrossRef CAS PubMed; (b) M. Onoda, T. Uchiyama, K. Mawatari, K. Kaneko and K. Nakagomi, Anal. Sci., 2006, 22, 815 CrossRef CAS PubMed.
- (a) M. Abo, Y. Urano, K. Hanaoka, T. Terai, T. Komatsu and T. Nagano, J. Am. Chem. Soc., 2011, 133, 10629 CrossRef CAS PubMed; (b) M. Abo, R. Minakami, K. Miyano, M. Kamiya, T. Nagano, Y. Urano and H. Sumimoto, Anal. Chem., 2014, 86, 5983 CrossRef CAS PubMed; (c) K. Zhang, W. Dou, P. Li, R. Shen, J. Ru, W. Liu, Y. Cui, C. Chen, W. Liu and D. Bai, Biosens. Bioelectron., 2015, 64, 542 CrossRef CAS PubMed.
- (a) A. R. Lippert, T. Gschneidtner and C. J. Chang, Chem. Commun., 2010, 46, 7510 RSC; (b) Y. Hitomi, T. Takeyasu, T. Funabiki and M. Kodera, Anal. Chem., 2011, 83, 9213 CrossRef CAS PubMed; (c) D. Song, J. M. Lim, S. Cho, S.-J. Park, J. Cho, D. Kang, S. G. Rhee, Y. You and W. Nam, Chem. Commun., 2012, 48, 5449 RSC; (d) Y. Hitomi, T. Takeyasu and M. Kodera, Chem. Commun., 2013, 49, 9929 RSC.
- (a) F. Yu, P. Li, P. Song, B. Wang, J. Zhao and K. Han, Chem. Commun., 2012, 48, 4980 RSC; (b) M. Kaur, D. S. Yang, K. Choi, M. J. Cho and D. H. Choi, Dyes Pigm., 2014, 100, 118 CrossRef CAS; (c) Y.-X. Liao, K. Li, M.-Y. Wu, T. Wu and X.-Q. Yu, Org. Biomol. Chem., 2014, 12, 3004 RSC.
- E. A. Veal, A. M. Day and B. A. Morgan, Mol. Cell, 2007, 26, 1 CrossRef CAS PubMed.
- S. L. Hempel, G. R. Buettner, Y. Q. O'Malley, D. A. Wessels and D. M. Flaherty, Free Radical Biol. Med., 1999, 27, 146 CrossRef CAS PubMed.
- M. G. Choi, J. O. Moon, J. Bae, J. W. Lee and S.-K. Chang, Org. Biomol. Chem., 2013, 11, 2961 CAS.
- Z. Wu, Z. Li, L. Yang, J. Han and S. Han, Chem. Commun., 2012, 48, 10120 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12984f |
| This journal is © The Royal Society of Chemistry 2016 |