DOI:
10.1039/C6RA12975G
(Paper)
RSC Adv., 2016,
6, 77515-77524
Enhanced corrosion inhibition properties of carboxymethyl hydroxypropyl chitosan for mild steel in 1.0 M HCl solution
Received
19th May 2016
, Accepted 1st August 2016
First published on 1st August 2016
Abstract
Synthesized carboxymethyl hydroxypropyl chitosan (CHPCS) containing both carboxymethyl and hydroxypropyl groups was investigated as a corrosion inhibitor for mild steel in 1.0 M HCl solution using weight loss, open circuit potential (OCP), potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. It was found that the compound behaved like a mixed-typed corrosion inhibitor that simultaneously inhibited the anodic dissolution and hydrogen evolution reactions. The compound inhibited corrosion at a low concentration and reached an inhibition efficiency of 95.3% in 1000 ppm (by weight). It was also found that the equivalent circuit model of one time constant was used during EIS analysis. The absorption of CHPCS on the metallic surface obeyed the Langmuir isotherm with a negative value of the free energy of adsorption ΔG. Scanning electron microscopy (SEM) was carried out to corroborate the test results. The potential of zero charge revealed that the corrosion inhibition mechanism involved physisorption and chemisorption processes.
1. Introduction
Mineral acids (HCl and H2SO4) are widely used to remove iron oxide and rust in industrial processes such as pickling, descaling and oil well acidizing.1–4 Aggressive acids accelerate the rate of metal dissolution and result in failure of the material. A useful method to mitigate the corrosion of metals and alloys in such environments is the addition of suitable inorganic and organic corrosion inhibitors to the solution in contact with the metallic surface. However, many inorganic compounds containing phosphate, chromate, tungstate or other heavy metal ions are under strict restriction in the international community. At the same time, unfortunately, many organic inhibitors containing oxygen, nitrogen and/or sulfur groups that are adsorbed on the metallic surface and hinder the processes of corrosion have been reported to be toxic, environmentally unfriendly and non-biodegradable, which represents a potential threat to humans.5–17
For safety and environmentally friendly purposes, the development of non-toxic and effective inhibitors (such as natural products extracted from plants and animals) is considered to be very important and desirable.18–21 Chitosan is fully or partially deacetylated from chitin (the second most abundant natural resource on Earth) and is considered to be a biodegradable compound.22,23 It is an attractive material to apply as a green corrosion inhibitor due to its electron-rich hydroxyl and amino groups, which can offer the possibility of ionic interactions with the metallic surface; these electrons are donated to empty or partially occupied Fe orbitals via coordinate bonds.24
The application of chitosan in water is limited due to its poor solubility. Chitosan derivatives were investigated for corrosion inhibition in previous studies. For example, Cheng has reported the inhibition effects of carboxymethyl chitosan (CMCT), Cu2+, and CMCT + Cu2+ mixture on the corrosion of mild steel in 1.0 M HCl using weight loss measurements and electrochemical techniques.25 Acetyl thiourea chitosan polymer (ATUCS) has been reported to be an effective corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. The effects of temperature, concentration of the corrosion inhibitor and immersion time were investigated.26 Furthermore, Umoren24 and Sangeetha27 have investigated the role of synthetically-derived chitosan in HCl for mild steel corrosion using chemical, electrochemical and surface analytical techniques. Their studies are efficient and productive. However, to the best of our knowledge, the corrosion inhibition application of CHPCS, which is less expensive, has easier preparation methods than the chitosan derivatives mentioned above and may have better corrosion inhibition properties than chitosan, hydroxypropyl chitosan or carboxymethyl chitosan (CCS), has not been reported. Chen et al.28 synthesized and characterized hydroxypropyl carboxymethyl chitosan. However, no applications for hydroxypropyl carboxymethyl chitosan were mentioned in their study. Our present work examines the corrosion inhibition capability of CHPCS synthesized from HPCS for mild steel in 1.0 M HCl solution using weight loss measurements, electrochemical techniques and adsorption isotherms. Meanwhile, the morphological changes of the corroding steel surface in the absence and presence of chitosan were observed by scanning electron microscopy (SEM).
2. Experimental
2.1 Materials
Hydroxypropyl chitosan (HPCS, MA: 4 × 105 to 6 × 105, degree of substitution: 2.05) and chloroacetic acid (CA) were purchased from Beijing Chem. CO.; hydrochloric acid, sodium hydroxide, ethyl alcohol, acetone and glacial acetic acid were applied by Sinopharm Chemical Reagent CO., Ltd. Q235 mild steel (composition in wt%, C: 0.14 to 0.22, Mn: 0.30 to 0.65, Si: 0.3, S: 0.050, P: 0.045) electrodes (10 mm × 10 mm × 5 mm) were polished sequentially with 400 grit, 800 grit, 1500 grit and 2000 grit emery papers and then degreased in acetone and ethanol prior to immersion in 1.0 M HCl solution for electrochemical impedance spectroscopy (EIS) and Tafel polarization measurements. Q235 standardized test panels (50 mm × 25 mm × 2 mm) were used for weight loss measurements.
2.2 Synthesis of CHPCS
CHPCS was synthesized according to the relevant reference (as shown in Fig. 1).28 HPCS (4.0 g) was dissolved in ultrapure water (50 mL) and then added to a three-necked flask equipped with a mechanical stirrer, a water-cooled condenser and a nitrogen inlet and outlet. After stirring and alkalization for 1 h, the CA solution (7.56 g CA + 17.0 mL H2O) was added dropwise to the reaction mixture, which was refluxed for 6 h at 60 °C. Then, the mixture was neutralized by the addition of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v glacial acetic acid and precipitated with acetone. The crude product was washed with acetone five times and dried in a vacuum drying oven at 50 °C for 24 h.
1 v/v glacial acetic acid and precipitated with acetone. The crude product was washed with acetone five times and dried in a vacuum drying oven at 50 °C for 24 h.
 |
| | Fig. 1 Synthetic route of carboxymethyl hydroxypropyl chitosan. | |
2.3 Fourier-transform infrared (FT-IR), nuclear magnetic resonance (1H NMR) and degree of substitution (DS) analysis
FT-IR spectra were recorded using a Nicolet IS10 Spectrometer, United States, in the frequency range of 4000 to 400 cm−1. 1H NMR spectra were recorded using an AVANCE III 600 MHz spectrometer in D2O (Bruker, Germany). The FT-IR spectrum of CHPCS was obtained at 298 K to certify if carboxyl existed in the compound and the 1H NMR spectrum was to determine the assignment of hydrogen elements. The DS value of CHPCS was calculated from the 1H NMR spectra.28
2.4 Electrochemical measurements
The electrochemical measurements of the corrosion inhibitors were performed on PARSTAT 2273 using a typical three-electrode system consisting of Q235 mild steel as the working electrode (WE, 1 cm2), a platinum film as the counter electrode (CE, 4 cm2), and a saturated calomel electrode (SCE) as the reference electrode. The working electrode was immersed in the test solution for 1 h until a steady potential (OCP) was reached prior to measurement. The potential values with immersion time were recorded. EIS measurements were performed in the frequency range from 105 to 10−1 Hz and with an amplitude of 10 mV. Tafel polarization curves of all the samples were obtained by automatically changing the electrode potential from −250 mV to +250 mV vs. the OCP at a constant sweep rate of 0.5 mV s−1 when the ranges of change of the OCP values were less than 10 μV s−1. All experiments were carried out at a temperature of 25 ± 1 °C. The electrochemical data were analyzed using the electrochemical software ZSimpWin. The inhibition efficiency was obtained from the following equations:30–32| |
 | (1) |
| |
 | (2) |
where Rct and R′ct are the charge transfer resistance in the presence and absence of the inhibitors, and icorr and i′corr are the corrosion current density of the uninhibited and inhibited specimens.
2.5 Weight loss measurements
The carbon steel sheets of 50 mm × 25 mm × 2 mm were washed with double distilled water, rinsed with ethanol and acetone, and then dried at room temperature (25 °C). After being weighed accurately, two parallel specimens were suspended in beakers containing 2000 mL of 1.0 M HCl solution with different concentrations of the tested inhibitors for 24 h. All the strong acid solutions were open to air. After each immersion time, the specimens were removed, washed with washing liquid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v H2O and concentrated hydrochloric acid +3.5 g L−1 hexamethylene tetramine) for 10 min at 25 °C, dried, and weighed accurately. The weight loss measurements were carried out in triplicate.
1 v/v H2O and concentrated hydrochloric acid +3.5 g L−1 hexamethylene tetramine) for 10 min at 25 °C, dried, and weighed accurately. The weight loss measurements were carried out in triplicate.
2.6 SEM observation
The surfaces of the metal samples were investigated after the cleaning procedure described above by SEM immediately after the end of the weight loss experiments. For the electron microscopic investigations, a Hitachi TM-1000 scanning electron microscope was used. The measurements for the microscopic investigations were performed at an acceleration voltage of 10 kV and a working distance of 10 mm.
3. Results and discussion
3.1 FT-IR analysis
The FT-IR spectra of HPCS and CHPCS are shown in Fig. 2. The absorption peak around 3245 cm−1 was assigned to the –OH in the HPCS. The broad peak around 3100 to 3300 cm−1 was assigned to the associated –OH in the HPCS and CHPCS. The C–H stretching vibration absorption bands around 3000 cm−1 were assigned to –CH3 and –CH2; in addition, C–H asymmetric and symmetric deformation vibration absorption peaks at 1450 and 1387 cm−1 appeared and were attributed to –CH3 and –CH2. The new absorption peaks at 1605.4 and 1416.2 cm−1 corresponded to the absorption peaks of the asymmetric stretching vibrations and symmetric stretching vibrations, respectively, indicating the existence of –COOH in the CHPCS.28 The absorption peaks occurring at 1380 cm−1 were attributed to the scissoring vibration of –CH3. This demonstrates the existence of the hydroxypropyl group. The C–O–C stretching vibration absorption peak (around 1150 cm−1) was attributed to the hydroxypropyl groups of HPCS and HCPCS.
 |
| | Fig. 2 FTIR spectra of HPCS (a) and CHPCS (b). | |
It can be seen in Fig. 3 that the 1H NMR spectrum contains peaks at δ = 1.15 ppm (d, 3H, −CH3) and δ = 2.21 ppm (s, 3H, −CH3) for the methyl hydrogens in the CHPCS. Peaks at δ = 3.0 to 4.0 ppm were attributed to the hydrogens of the methylene and methane groups on the sugar ring and the hydroxypropyl groups. Peaks at δ = 4.32 (s, 2H) and 4.55 (t, 2H) ppm were assigned to the methylene protons of N(CH2COOH)2 and NHCH2COOH, respectively. The peak at δ = 4.65 (s, 2H) was assigned to the methylene protons of OCH2COOH. The peak around δ = 5.4 was attributed to the hydrogen in the methane on the sugar ring.
 |
| | Fig. 3 1H-NMR spectrum of CHPCS. | |
According to (ref. 28), the DS of CHPCS can be calculated via the formula:
| |
 | (3) |
where
IH10,
IH12,
IH13 are the peak areas of H10 (–OCH
2COOH), H12 (–NHCH
2COOH) and H13 (–N(CH
2COOH)
2).
IH1 is the peak area of H1. The degree of substitution for CHPCS was 1.25.
3.2 SEM observation
Scanning electron micrographs (SEM) of the surface of mild steel immersed in 1.0 M HCl without and with the inhibitors are shown in Fig. 4. Fig. 4a reveals some relevant surface characteristics of the mild steel samples before exposure to the corrosive solution. However, a highly corroded metal surface can be observed in Fig. 4b, owing to the absence of CHPCS. The presence of CHPCS as an inhibitor provided a cleaner and smoother metal surface; after that, as the concentration of corrosion inhibitor increased, the metallic surface appeared to be increasingly smooth (Fig. 4c–f). This demonstrated the good protective potential of CHPCS to act as a corrosion inhibitor for mild steel in acid media.
 |
| | Fig. 4 SEM images for (a) unexposed mild steel; (b) exposed mild steel in blank. Solution; and exposed mild steel in 1.0 M HCl containing (c) 75 ppm (d) 200 ppm (e) 600 ppm and (f) 1000 ppm after 24 h immersion at 298 K. | |
3.3 Electrochemical experiments
3.3.1 Open circuit potential and potentiodynamic polarization measurements. The OCP values (vs. SCE) of the samples during 1 h immersion time were measured in the test solution. The relationship between the OCP and the immersion time is shown in Fig. 5. As shown in Fig. 5, the working electrodes reach a steady state after 1 h immersion, and the OCP values show little change (less than 1 mV). The initial value of the blank sample was −0.524 V, and it became almost constant around −0.483 V. Increasing OCP indicates the initiation and propagation of corrosion, whereas relatively stable OCP values imply a steady state between the progress of corrosion and the deposit of corrosion products.29 In the presence of the inhibitor solutions, the OCP values shifted towards more positive potentials during the immersion. This can be explained by the absorption of inhibitors on the metal surface (Fig. 10).
 |
| | Fig. 5 Open circuit potential of mild steel in the presence of various concentrations of corrosion inhibitors at 25 °C in 1.0 M HCl. | |
After a short immersion time (500 s), the OCP values increased with immersion time. The fluctuating period from 500 s to 3300 s could be could be interpreted as the dynamic process by adsorption and desorption of the inhibitor molecules on the metal surface. At the end of the immersion (3300 s to 3600 s), the values tended to stabilize, demonstrating that the adsorption and desorption of inhibitor molecules had reached a dynamic balance.
Potentiodynamic polarization reflects the impact of a corrosion inhibitor on the electrode behavior of carbon steel in 1.0 M HCl. The potentiodynamic polarization curves in 1.0 M HCl in the presence of different concentrations of HPCS and CHPCS at 25 °C are shown in Fig. 6. The electrochemical parameters determined from the polarization curves, including corrosion potential (Ecorr), anode Tafel slope (βa), cathodic Tafel slope (βc), corrosion current density (icorr) and inhibition efficiency (η), are depicted in Table 1. According to published studies,19,33–35 if the change in Ecorr value is (i) more than 85 mV in an inhibited system with respect to an uninhibited system, the inhibitor could be defined as anodic or cathodic type; if it is (ii) less than 85 mV, it could be defined as mixed-type (85 mV). As can be obviously seen in Fig. 6, the inhibitor can be recognized as mixed-type, indicating that the process of the anode corrosion reaction was nearly equal to the process of cathode inhibition. In the cathodic domain, the nearly constant polarization curves in the absence and in the presence of CHPCS in Fig. 6 show that the hydrogen evolution mechanism did not change with the addition of CHPCS and that the reduction of H+ on the mild steel surface occurred mainly through a charge transfer mechanism.36–38 The adsorbed inhibitor molecules only blocked the active sites of hydrogen evolution on the metal surface. In the anodic domain, a decrease in the current densities occurred after the addition of CHPCS, and then, the inhibitor started to desorb from metallic surface for potentials higher than −320 mV. The inhibitor molecules deviated from the metal surface with the dissolution of metal anodes, which is usually defined as an anodic desorption process.39–42
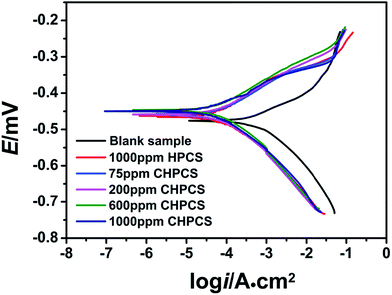 |
| | Fig. 6 Potentiodynamic polarization curves of mild steel in presence of various concentrations of corrosion inhibitors at 25 °C in 1.0 M HCl. | |
Table 1 Potentiodynamic polarization parameters for the corrosion of mild steel in 1.0 M HCl solution containing various concentrations of HPCS and CHPCS at 25 °C after 1 h immersion
| Inhibitor |
Concentration (ppm by weight) |
icorr (mA cm−2) |
Ecorr (mV vs. SCE) |
βa (mV dec−1) |
βc (mV dec−1) |
η (%) |
| Blank |
0.0 |
1.10 |
−480.1 |
81.3 |
−117.1 |
— |
| HPCS |
75 |
0.123 |
−475.4(−475.2) |
63.0 |
−116.8 |
88.9 |
| 200 |
0.122 |
−474.8(−477.2) |
55.6 |
−98.1 |
89.0 |
| 600 |
0.112 |
−473.6(−473.7) |
85.2 |
−139.0 |
90.0 |
| 1000 |
0.101 |
−470.4 |
81.9 |
−109.7 |
91.0 |
| CHPCS |
75 |
0.117 |
−469.2 |
71.0 |
−135.1 |
89.3 |
| 200 |
0.113 |
−466.7 |
76.0 |
−135.1 |
89.9 |
| 600 |
0.096 |
−465.2 |
63.3 |
−86.8 |
91.2 |
| 1000 |
0.052 |
−459.1 |
72.3 |
−90.0 |
95.3 |
It is obvious from Fig. 6 and Table 1 that both the anodic and cathodic current values decreased considerably in the inhibitor solutions compared with the uninhibited solution. The synthesized inhibitor CHPCS exhibited more corrosion inhibition of mild steel than HPCS and the solution with no inhibitor, according to the data in Table 1. This evidence shows that the corrosion potential (Ecorr) was higher and the corrosion current density (icorr) was smaller than that of the other specimens. The inhibition efficiency (η) of mild steel with CHPCS was also higher than that of HPCS. The values of η were rated as (1000 ppm) > (600 ppm) > (200 ppm) > (75 ppm).
3.3.2 EIS measurements. The Nyquist and Bode plots of mild steel obtained in 1.0 M HCl solution with various concentrations of CHPCS (75 ppm, 200 ppm, 600 ppm, and 1000 ppm) at 25 °C are given in Fig. 7 and 8. The Nyquist parameters obtained by ZSimpWin impedance analysis software are presented in Table 2. It is clear that all the Nyquist plots for mild steel in the test solution consist of a capacitive loop at high frequency. The impedance and phase angle at the high frequency range represent an adsorption phenomenon, and a charge transfer reaction occurred at low frequency.43 The phase Bode plots (phase angle as a function of the logarithm of the frequency f) coincide with one time constant and the impedance Bode plots (impedance module value as a function of the logarithm of the frequency f) show the impedance of a double layer. This indicates that the corrosion inhibition type is monolayer absorption. However, the obtained Nyquist plots, shown in Fig. 7, are not perfect semicircles, which was due to the non-homogeneity, surface roughness and porosity of the electrode surface.44,45
 |
| | Fig. 7 Nyquist plots of mild steel electrode obtained in 1.0 M HCl solution with various concentrations of corrosion inhibitors at 25 °C. | |
 |
| | Fig. 8 Bode plots of mild steel electrode obtained in 1.0 M HCl solution of corrosion inhibitors with various concentrations at 25 °C (a) Bode–|Z| plots (b) Bode–(phase) plots. | |
Table 2 Electrochemical impedance parameters for the corrosion of mild steel in 1.0 M HCl solution containing various concentrations of HPCS and CHPCS at 25 °C after 1 h immersion
| Inhibitor |
Concentration (ppm by weight) |
Rs (Ω cm−2) |
Rct (Ω cm−2) |
Y0 (μF cm−2) |
n |
Cdl (μF cm−2) |
η (%) |
| Blank |
0.0 |
0.8405 |
30.8 |
285.2 |
0.908 |
191.8 |
— |
| HPCS |
75 |
1.429 |
217.4 |
245.8 |
0.887 |
148.3 |
85.8 |
| 200 |
1.457 |
219.1 |
212.3 |
0.895 |
132.8 |
86.1 |
| 600 |
1.268 |
229.5 |
205.1(249.2) |
0.885(0.855) |
130.1(130.3) |
86.6 |
| 1000 |
1.319 |
245.8 |
198.2 |
0.901 |
129.6 |
87.5 |
| CHPCS |
75 |
0.893 |
260.9 |
227.2 |
0.838 |
116.2 |
88.1 |
| 200 |
0.7166 |
263.8 |
141.5 |
0.917 |
102.2 |
88.3 |
| 600 |
0.736 |
275.5 |
135.2(184.3) |
0.890(0.870) |
96.5(107.6) |
88.9 |
| 1000 |
0.636 |
371.4 |
93.66 |
0.915 |
69.4 |
91.7 |
The electrochemical process was analyzed by the equivalent circuit method, and the corresponding physical model is presented in Fig. 9. In Fig. 9, Rs is the solution resistance. CPE (Constant phase element) was employed instead of the “ideal” capacitance. Rct is the charge transfer resistance, and the impedance of the CPE was expressed as:46–48
| |
 | (4) |
where
Y0 represents the frequency independent admittance of CPE, j is the imaginary unit and
ω is the angular frequency.
N (CPE exponent) is related to the angle of rotation of a purely capacitive line on the complex plane plot, which is generally close to 1. If
n = 1, the CPE is equivalent to the pure capacitance; if
n = 0.5, the CPE is equivalent to the Warburg impedance; and if
n = −1, the CPE is equivalent to the inductance.
49,50 At this point, the values of
Y0 and
n were obtained from the ZSimpWin software, and
Cdl could be calculated using
eqn (5):
51,52| | |
Cdl = Y0(ω)n−1 = Y0(2πfmax)n−1
| (5) |
where
fmax represents the frequency at which the imaginary value reached a maximum on the Nyquist plots in
Fig. 7.
 |
| | Fig. 9 The equivalent circuit used to fit the obtained impedance spectra for mild steel in the absence and presence of inhibitors. | |
It is apparent from Table 2 that the Rs values were similar to each other. The CPE exponents were between 0.85 and 0.95, which indicates a rough environment on the metal surface. The values of Rct increased and Y0 decreased with the increasing concentration of CHPCS solution.49 The increasing Rct was possibly due to the formation of a protective layer on the metal surface, which significantly retarded the metal dissolution and the charge transfer. On the other hand, there was a relatively wider margin of decrease of the Cdl value with the addition of CHPCS, also, the Cdl decreased with increasing inhibitor concentration, which is attributed to the thicker protective layer.51 According to (ref. 52), this indicates that the thickness of the adsorbed inhibitor molecules increased with decreasing Cdl. Thus, the result of the Cdl value was consistent with the EIS measurements. Furthermore, the highest effect was observed at 1000 ppm, which is in good agreement with the results obtained from the potentiodynamic polarization measurements.
3.4 Weight loss measurements
The effect of the addition of different concentrations of HPCS and CHPCS on the corrosion of mild steel in 1.0 M HCl solution was studied by weight loss measurements at 25 °C after a 24 h immersion period. The values of corrosion rate (v) and inhibition efficiency (η) with and without inhibitors were demonstrated in Table 3. The corrosion rate and inhibition efficiency were calculated by the following equations.53| |
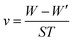 | (6) |
| |
 | (7) |
where W and W′ are the weights of the mild steel samples without and with inhibition, respectively. S is the total surface test area of the sample, and T is the immersion time (24 h). v and v′ are the corrosion rates in the absence and presence of inhibitors, respectively.
Table 3 Corrosion parameters obtained from weight loss measurements for mild steel in 1.0 M HCl in the absence and presence of various concentrations of corrosion inhibitors at 25 °C after 24 h immersion
| Inhibitor |
Concentration (ppm by weight) |
v (mg cm−2 h−1) |
η (%) |
| Blank |
— |
0.318 |
— |
| HPCS |
75 |
0.046 |
85.5 |
| 600 |
0.044 |
86.2 |
| 1000 |
0.042 |
86.8 |
| 1000 |
0.039 |
87.7 |
| CHPCS |
75 |
0.039 |
87.7 |
| 200 |
0.037 |
88.4 |
| 600 |
0.033 |
89.6 |
| 1000 |
0.029 |
90.9 |
Indeed, from Table 3, it is revealed that the corrosion rates decreased and the inhibition efficiencies improved with increasing concentrations of HPCS and CHPCS. Additionally, the inhibition efficiency of CHPCS was higher than that of HPCS at the same concentration, which suggested that the additional functional groups in CHPCS, such as carboxymethyl, were involved in the corrosion inhibition process. The highest η of 90.9% was acquired in the presence of 1000 ppm CHPCS, which was consistent with the results obtained by EIS and the potentiodynamic polarization curves.
3.5 Adsorption isotherms and corrosion inhibition mechanism
3.5.1 Adsorption isotherms. Adsorption isotherm experiments were carried out to obtain more insight into the corrosion inhibition mechanism.54 Generally, if there are no other reactions except the adsorption and desorption processes of the corrosion inhibitor on the metal surface, the coverage of absorption (θ) has an equilibrium relationship with the concentration of the corrosion inhibitor (C). In the simplest case, the adsorbed layer is a monomolecular layer; that is to say, interaction between the adsorption particles does not occur on the metal surface. The surface coverage value (θ) is equal to the inhibition efficiency (η). The inhibition efficiency values (η) for various concentrations of the inhibitors in the test solution, obtained by EIS measurements, are illustrated in Table 2. Several adsorption isotherms were employed here, including the Bockris–Swinkeles, Temkin, and Frumkin adsorption isotherm models.55 The linear relationships of C/θ versus C, as shown in Fig. 10, confirmed that the suitable adsorption isotherm model was the Langmuir model. K obeyed the Langmuir adsorption isotherm. The equation associated with the Langmuir model can be described as follows:56| |
 | (8) |
where K is the adsorption equilibrium constant, which is related to ΔG, expressed by the following equation:57| |
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(55.5 × K) ln(55.5 × K)
| (9) |
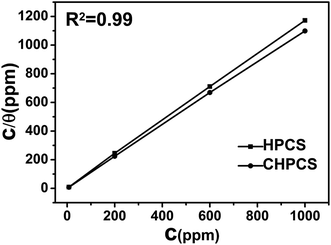 |
| | Fig. 10 Langmuir isotherms for the adsorption of corrosion inhibitors on the surface of mild steel at 298 K. | |
R is the universal gas constant, 8.314 J K−1 mol−1, and T is the temperature in K. The value of 55.5 is the concentration of water in solution expressed in moles per litre. ΔG can characterize the interaction of adsorption molecules with a metal surface. All of calculated data were shown in Table 4. The calculated ΔG values indicated that the adsorption of the corrosion inhibitor on the metal surface was a spontaneous process, and the inhibition efficiency associated with solution concentration increased with increasingly negative value of ΔG. Generally speaking, ΔG values around or higher than −20 kJ mol−1 are in accord with physisorption, while values lower than −40 kJ mol−1 are defined as chemisorption and values between −20 kJ mol−1 and 40 kJ mol−1 involve mixed adsorption.58 In this case, it was noted that the corrosion inhibitor CHPCS was consistent with a mixed corrosion inhibitor, according to the values from Table 1–3.
Table 4 Equilibrium constants and standard Gibbs free energies of adsorption of mild steel in 1.0 M HCl with various concentrations of corrosion inhibitors at 25 °C (298 K)
| Inhibitor |
Concentration (ppm by weight) |
Linear regression coefficient (R2) |
Kads (103 M−1) |
ΔGoads (kJ mol−1) |
| HPCS |
75 |
0.99 |
5.81 |
−31.4 |
| 200 |
0.99 |
9.17 |
−32.5 |
| 600 |
0.99 |
22.6 |
−34.7 |
| 1000 |
0.99 |
68.0 |
−37.5 |
| CHPCS |
75 |
0.99 |
14.1 |
−33.6 |
| 200 |
0.99 |
14.1 |
−33.6 |
| 600 |
0.99 |
42.0 |
−36.3 |
| 1000 |
0.99 |
270.4 |
−38.9 |
3.5.2 The potential of zero charge (PZC) and corrosion inhibition mechanism of CHPCS. As mentioned above, the compound CHPCS is a mixed corrosion inhibitor, involving physisorption and chemisorption. The schematic diagram of the adsorption mechanism of CHPCS on Q235 mild steel in 1.0 M HCl solution is shown in Fig. 11. As a result of the HCl in solution, the amino groups occurred in protonated form. Thus, there were two adsorption forces in the system: (a) the physisorption force derived from the electrostatic interaction between the protonated amine groups and the negatively charged metal surface created by Cl− anions; (b) the chemisorption force derived from the interaction between the lone electron pairs of nitrogen atoms or oxygen atoms in the amino and carboxyl (hydroxyl) groups and the empty 3d orbitals of iron atoms on the metallic surface.51 It is worth noting that the –COOH group in CHPCS (seen from Fig. 9) offered extra chemisorption. The improvement of the corrosion inhibition efficiency was due to this extra chemisorption. In addition, we examined the corrosion products for CHPCS by FTIR. From Fig. 12, we can see a peak at 3200 cm−1, assigned to –OH, and peaks at 1590 cm−1 and 1420 cm−1, attributed to –COOH; this proves that –COOH offered extra chemisorption. The mechanism analysis agreed well with the experimental results.
 |
| | Fig. 11 Schematic of the adsorption mechanism of HPCS and CHPCS on mild steel in 1.0 M HCl solution: (a) physical adsorption, (b) chemical adsorption. | |
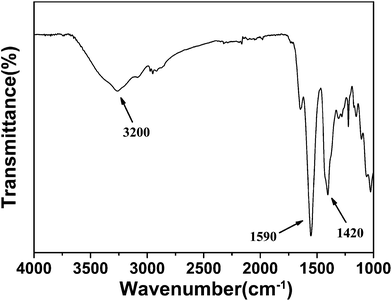 |
| | Fig. 12 FTIR spectrum of the corrosion products of CHPCS. | |
To certify the mechanism proposed above, we tested the potential of zero charge (PZC) of corrosion inhibition in 1.0 M HCl solution with a concentration of 1000 ppm. The potential of zero charge is a state where the electric charge density on a surface is zero. At the stationary potential, the metal surface will be positively or negatively charged, which will influence the adsorption of corrosion inhibitor molecules. The data of EPZC are determined by measuring the maximum Rp values or minimum Cdl values vs. the potential.59,60
Fig. 13 shows the Rp values vs. potential (vs. SCE); the acquired curve appears parabolic in shape. The potential with maximum Rp was −0.540 V, which is the EPZC. It was observed that the Eocp was −0.459 V during the experiments. The Er (Er = Eocp − EPZC) was +0.081 V, which indicates that the metallic surface carried the positive charges. Thus, Cl ions would be first absorbed on the metallic surface; then, protonated corrosion inhibitor molecules would combine with Cl− through electrostatic attraction to form barrier layers. In addition, coordinate bonds formed via N, O atoms and d unoccupied molecular orbitals also existed on the metallic surface.
 |
| | Fig. 13 The plot of Rp vs. potential in 1.0 M HCl solution of corrosion inhibitor containing 1000 ppm CHPCS. | |
4. Conclusion
CHPCS has been synthesized and evaluated for its enhanced inhibitory performance on mild steel in 1.0 M HCl solution. From the obtained results, the following conclusions can be made:
(1) From the electrochemical experiments and weight loss measurements, CHPCS inhibits mild steel corrosion better than HPCS in 1.0 M HCl solution.
(2) CHPCS can be used as an efficient inhibitor for mild steel corrosion at very low concentrations. The inhibition efficiency increases with increasing corrosion inhibitor concentration. The optimal corrosion inhibition condition is 1000 ppm by weight, which is attributed to the high inhibition solution concentration and extra chemisorption offered by –COOH.
(3) The potential of zero charge technique reveals that CHPCS inhibits both anodic and cathodic reactions by adsorption on the mild steel surface; hence, it behaves like a mixed-type inhibitor.
(4) The adsorption model of CHPCS obeys the Langmuir adsorption isotherm, and the negative value of the Gibbs free energy of adsorption (ΔG) indicates a strong interaction between the inhibitor molecules and the metallic surface.
Acknowledgements
The authors gratefully acknowledge financial support by the National Natural Science Foundation of China (No. 201005028-1 and 41276074) and the Postdoctoral Science Foundation of Qingdao.
References
- A. K. Singh and M. A. Quraishi, Corros. Sci., 2010, 52, 152–160 CrossRef CAS.
- G. Golestani, M. Shahidi and D. Ghazanfari, Appl. Surf. Sci., 2014, 308, 347–362 CrossRef CAS.
- A. Khamis, M. M. Saleh and M. I. Awad, Corros. Sci., 2013, 66, 343–349 CrossRef CAS.
- I. Ahamad, R. Prasad and M. A. Quraishi, Corros. Sci., 2010, 52, 933–942 CrossRef CAS.
- A. V. Shanbhag, T. V. Venkatesha, R. A. Prabhu, R. G. Kalkhambkar and G. M. Kulkarni, J. Appl. Electrochem., 2007, 38, 279–287 CrossRef.
- M. Bobina, A. Kellenberger, J.-P. Millet, C. Muntean and N. Vaszilcsin, Corros. Sci., 2013, 69, 389–395 CrossRef CAS.
- R. Yıldız, A. Döner, T. Doğan and İ. Dehri, Corros. Sci., 2014, 82, 125–132 CrossRef.
- H. Bentrah, Y. Rahali and A. Chala, Corros. Sci., 2014, 82, 426–431 CrossRef CAS.
- S. A. Ali, H. A. Al-Muallem, M. T. Saeed and S. U. Rahman, Corros. Sci., 2008, 50, 664–675 CrossRef CAS.
- I. Frateur, L. Lartundo-Rojas, C. Méthivier, A. Galtayries and P. Marcus, Electrochim. Acta, 2006, 51, 1550–1557 CrossRef CAS.
- B. D. Mert, A. O. Yüce, G. Kardaş and B. Yazıcı, Corros. Sci., 2014, 85, 287–295 CrossRef CAS.
- R. A. Prabhu, T. V. Venkatesha, A. V. Shanbhag, G. M. Kulkarni and R. G. Kalkhambkar, Corros. Sci., 2008, 50, 3356–3362 CrossRef CAS.
- P. Roy, A. Pal and D. Sukul, RSC Adv., 2014, 4, 10607–10613 RSC.
- N. O. Obi-Egbedi, I. B. Obot and A. O. Eseola, Arabian J. Chem., 2014, 7, 197–207 CrossRef CAS.
- M. Yadav, R. Sinha, S. Kumar and T. Sarkar, RSC Adv., 2015, 5, 70832–70848 RSC.
- B. Xu, Y. Ji, X. Zhang, X. Jin, W. Yang and Y. Chen, RSC Adv., 2015, 5, 56049–56059 RSC.
- C. Verma, M. Quraishi, L. Olasunkanmi and E. E. Ebenso, RSC Adv., 2015, 5, 85417–85430 RSC.
- N. El Hamdani, R. Fdil, M. Tourabi, C. Jama and F. Bentiss, Appl. Surf. Sci., 2015, 357, 1294–1305 CrossRef CAS.
- S. S. Shivakumar and K. N. Mohana, Int. J. Corros., 2013, 2013, 1–13 CrossRef.
- V. Rajeswari, D. Kesavan, M. Gopiraman, P. Viswanathamurthi, K. Poonkuzhali and T. Palvannan, Appl. Surf. Sci., 2014, 314, 537–545 CrossRef CAS.
- A. Biswas, S. Pal and G. Udayabhanu, Appl. Surf. Sci., 2015, 353, 173–183 CrossRef CAS.
- B. Balakrishnan, M. Mohanty, P. R. Umashankar and A. Jayakrishnan, Biomaterials, 2005, 26, 6335–6342 CrossRef CAS PubMed.
- R. Jayakumar, N. Nwe, S. Tokura and H. Tamura, Int. J. Biol. Macromol., 2007, 40, 175–181 CrossRef CAS PubMed.
- S. A. Umoren, M. J. Banera, T. Alonso-Garcia, C. A. Gervasi and M. V. Mirífico, Cellulose, 2013, 20, 2529–2545 CrossRef CAS.
- S. Cheng, S. Chen, T. Liu, X. Chang and Y. Yin, Electrochim. Acta, 2007, 52, 5932–5938 CrossRef CAS.
- A. M. Fekry and R. R. Mohamed, Electrochim. Acta, 2010, 55, 1933–1939 CrossRef CAS.
- Y. Sangeetha, S. Meenakshi and C. S. Sundaram, Carbohydr. Polym., 2016, 136, 38–45 CrossRef CAS PubMed.
- Q. Chen, X. Han, S. Peng, J. He, M. Deng, Y. Zhao and B. Jiang, J. Appl. Polym. Sci., 2014, 131, 40460 Search PubMed.
- R. Yildiz, An electrochemical and theoretical evaluation of 4,6-diamino-2-pyrimidinethiol as a corrosion inhibitor for mild steel in HCl solutions, Corros. Sci., 2015, 90, 544 CrossRef CAS.
- S. El-Egamy, Corros. Sci., 2008, 50, 928–937 CrossRef CAS.
- Q. Deng, N.-N. Ding, X.-L. Wei, L. Cai, X.-P. He, Y.-T. Long, G.-R. Chen and K. Chen, Corros. Sci., 2012, 64, 64–73 CrossRef CAS.
- C. Verma, M. Quraishi and E. Ebenso, Int. J. Electrochem. Sci., 2013, 8, 7401–7413 CAS.
- P. Bothi Raja and M. G. Sethuraman, Mater. Lett., 2008, 62, 1602–1604 CrossRef.
- Y. Yan, W. Li, L. Cai and B. Hou, Electrochim. Acta, 2008, 53, 5953–5960 CrossRef CAS.
- H. Ashassi-Sorkhabi, M. R. Majidi and K. Seyyedi, Appl. Surf. Sci., 2004, 225, 176–185 CrossRef CAS.
- H. Hamani, T. Douadi, M. Al-Noaimi, S. Issaadi, D. Daoud and S. Chafaa, Corros. Sci., 2014, 88, 234–245 CrossRef CAS.
- M. Behpour, S. M. Ghoreishi, N. Soltani and M. Salavati-Niasari, Corros. Sci., 2009, 51, 1073–1082 CrossRef CAS.
- S. Issaadi, T. Douadi, A. Zouaoui, S. Chafaa, M. A. Khan and G. Bouet, Corros. Sci., 2011, 53, 1484–1488 CrossRef CAS.
- A. Döner, R. Solmaz, M. Özcan and G. Kardaş, Corros. Sci., 2011, 53, 2902–2913 CrossRef.
- W. J. Lorenz and F. Mansfeld, Electrochim. Acta, 1986, 31, 467–476 CrossRef CAS.
- F. Bentiss, M. Bouanis, B. Mernari, M. Traisnel, H. Vezin and M. Lagrenée, Appl. Surf. Sci., 2007, 253, 3696–3704 CrossRef CAS.
- A. El Bribri, M. Tabyaoui, B. Tabyaoui, H. El Attari and F. Bentiss, Mater. Chem. Phys., 2013, 141, 240–247 CrossRef CAS.
- A. V. Benedeti, P. T. A. Sumodjo, K. Nobe, P. L. Cabot and W. G. Proud, Electrochim. Acta, 1995, 40, 2657–2668 CrossRef.
- M. Morad, Corros. Sci., 2000, 42, 1307–1326 CrossRef CAS.
- R. S. Gonçalves, D. S. Azambuja and A. M. S. Lucho, Corros. Sci., 2002, 44, 467–479 CrossRef.
- N. Labjar, M. Lebrini, F. Bentiss, N.-E. Chihib, S. El Hajjaji and C. Jama, Mater. Chem. Phys., 2010, 119, 330–336 CrossRef CAS.
- Z. Stoynov, Electrochim. Acta, 1990, 35, 1493–1499 CrossRef.
- C. Hsu and F. Mansfeld, Corrosion, 2001, 57, 747–748 CrossRef CAS.
- F. Bentiss, C. Jama, B. Mernari, H. E. Attari, L. E. Kadi, M. Lebrini, M. Traisnel and M. Lagrenée, Corros. Sci., 2009, 51, 1628–1635 CrossRef CAS.
- M. Özcan and İ. Dehri, Corros. Sci., 2012, 54, 201–204 CrossRef.
- S.-H. Yoo, Y.-W. Kim, K. Chung, N.-K. Kim and J.-S. Kim, Ind. Eng. Chem. Res., 2013, 52, 10880–10889 CrossRef CAS.
- K. Mallaiya, R. Subramaniam, S. S. Srikandan, S. Gowri, N. Rajasekaran and A. Selvaraj, Electrochim. Acta, 2011, 56, 3857–3863 CrossRef CAS.
- A. Hernández-Espejel, M. A. Domínguez-Crespo, R. Cabrera-Sierra, C. Rodríguez-Meneses and E. M. Arce-Estrada, Corros. Sci., 2010, 52, 2258–2267 CrossRef.
- P. Morales-Gil, G. Negrón-Silva, M. Romero-Romo, C. Ángeles-Chávez and M. Palomar-Pardavé, Electrochim. Acta, 2004, 49, 4733–4741 CrossRef CAS.
- M. Bouklah, B. Hammouti, M. Lagrenee and F. Bentiss, Corros. Sci., 2006, 48, 2831–2842 CrossRef CAS.
- R. Agrawal and T. Namboodhiri, Corros. Sci., 1990, 30, 37–52 CrossRef CAS.
- J. Cases and F. Villieras, Langmuir, 1992, 8, 1251–1264 CrossRef CAS.
- E. A. Noor and A. H. Al-Moubaraki, Mater. Chem. Phys., 2008, 110, 145–154 CrossRef CAS.
- A. Doner, E. A. Sahi, G. Kardas and O. Serindag, Investigation of corrosion inhibition effect of 3-[(2-hydroxy-benzylidene)-amino]-2-thioxo-thiazolidin-4-one on corrosion of mild steel in the acidic medium, Corros. Sci., 2013, 66, 278 CrossRef CAS.
- A. O. Yuce, B. D. Mert, G. Kardas and B. Yazıcı, Electrochemical and quantum chemical studies of 2-amino-4-methyl-thiazole as corrosion inhibitor for mild steel in HCl solution, Corros. Sci., 2014, 83, 310 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v glacial acetic acid and precipitated with acetone. The crude product was washed with acetone five times and dried in a vacuum drying oven at 50 °C for 24 h.
1 v/v glacial acetic acid and precipitated with acetone. The crude product was washed with acetone five times and dried in a vacuum drying oven at 50 °C for 24 h.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v H2O and concentrated hydrochloric acid +3.5 g L−1 hexamethylene tetramine) for 10 min at 25 °C, dried, and weighed accurately. The weight loss measurements were carried out in triplicate.
1 v/v H2O and concentrated hydrochloric acid +3.5 g L−1 hexamethylene tetramine) for 10 min at 25 °C, dried, and weighed accurately. The weight loss measurements were carried out in triplicate.







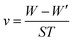


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(55.5 × K)
ln(55.5 × K)








