DOI:
10.1039/C6RA12934J
(Paper)
RSC Adv., 2016,
6, 72487-72499
Adsorptive removal of toxic metals and cationic dyes by magnetic adsorbent based on functionalized graphene oxide from water
Received
18th May 2016
, Accepted 25th July 2016
First published on 25th July 2016
Abstract
In this study, a new magnetic nanocomposite adsorbent was prepared from amino-silane functionalized graphene oxide (GO-APTS) and a copolymer of 2-acrylamido-2-methylpropanesulfonic acid (AMPS)/maleic anhydride (MA) [poly(AMPS-co-MA)], and adsorptive removal of Pb(II), Cu(II) and Co(II) and cationic dyes such as crystal violet (CV) and methylene blue (MB) from water was investigated. The magnetic adsorbent GO-APTS-poly(AMPS-co-MA)/Fe3O4 was characterized by the Fourier transform infrared spectroscopy (FT-IR), field emission scanning electron microscopy (FE-SEM), energy dispersive X-ray spectroscopy (EDS), X-ray diffraction (XRD), vibrating sample magnetometer (VSM), and thermogravimetric analysis (TGA). The effect of different factors on adsorption efficiency such as pH, adsorbent dosage, contact time, the concentration of adsorbates and temperature were investigated. The adsorption results fitted well with the Langmuir isotherm and pseudo-second-order kinetic model. The maximum adsorption capacity (Qm) was 310.10, 282.25, 238.35, 416.06 and 440.97 mg g−1 for Pb(II), Cu(II), Co(II), MB and CV, respectively. The adsorption was proposed to be chemisorption, spontaneous, and endothermic. The adsorbent was regenerated by several adsorptions/desorption processes. The prepared adsorbent can be highly efficient, magnetically separable and reusable for water purification.
1. Introduction
One of the worldwide serious problems to human life is contamination of water by heavy metal ions and dyes which are widely discharged from industries. Their accumulation in the living organisms can cause brain damage and mental retardation.1–3 Among different methods such as chemical precipitation,4 electrochemical treatment,5 ion exchange,6 membrane separation,7 liquid extraction,8 and reverse osmosis9 which have been employed for the removal of heavy metal ions and organic pollutants from water, the adsorption process is an effective, economical, and easily handled method for water purification.1 Many adsorbents including activated carbon, silica, metal oxides, and polymer resins have been developed.10 However, the problems of traditional adsorbents can be overcome by nano-size adsorbents with controlled functionalities, high surface area, and enhanced active sites. One of the highly efficient adsorbents for water treatment is carbon-based nanomaterials including carbon aerogels, carbon nanotubes (CNTs), and graphene.11 Graphene oxide (GO) is a nontoxic and inexpensive solid material containing various ionizable functional groups such as hydroxyl and carboxyl groups, which can exchange ions with metal ions or positively charged organic molecules and has been used as nanocarrier for delivery of various drugs.12–14 These groups improve GO sheets dispersion in polar solvents, enhance their miscibility with a polymer matrix and form a homogeneous colloidal suspension.15,16 Modification procedure is one of the important approaches to introduce more chelating functional groups into the structure and enhance the adsorption capacity of the adsorbents. Zhou et al. synthesized a new cellulose-based adsorbent by a facile modification route and used for removal of crystal violet dye from water. The results indicated 70.8% increasing in adsorption compared to native cellulose.17 A highly efficient adsorbent was prepared via modifying the cellulose with glycidyl methacrylate and diethylenetriamine pentaacetic acid for removal of cationic dyes from water.18 The grafting of the functionalized polymers onto the GO nanosheets is an easy method to enhance their miscibility within a polymer and adsorption properties due to the incorporation of more chelating groups.11,19,20 Recently, much attention has been paid to the embedding of magnetic (Fe3O4) nanoparticles in the matrix and the regeneration of adsorbent due to the easy separation from aqueous solution by an external magnet without contamination.21,22 Embedding magnetic nanoparticles in the matrix can also enhance the adsorption capacity of adsorbent due to the improvement in electrostatic interaction.23 A superparamagnetic GO–Fe3O4 hybrid composite has been developed and its potential applications in removing organic dyes from the polluted water were studied.24 It is believed that hybrids of the magnetic nanoparticles with high surface area and GO would have better functionalities and performances in the removal of contaminants from wastewater. There are reports in the literature for preparation of nanohybrids composed of sulfonated GO/Fe3O4 nanoparticles,25 and hydrogels of GO/polyethylenimine (PEI) as high adsorbents for dyes such as methylene blue and rhodamine B.26 Also, hierarchical core–shell manganese oxide nanocomposites (Fe3O4@MnO2 and Fe2O3@MnO2) were prepared via a facile hydrothermal process as adsorbent for wastewater treatment with good adsorption capability.27
In the present study, we aimed to synthesize a new magnetic functionalized adsorbent capable of removing metal ions and cationic dyes from water more effectively. The present magnetic adsorbent GO-APTS-poly(AMPS-co-MA)/Fe3O4 was prepared from amine-functionalized GO (GO-APTS) and a copolymer of 2-acrylamido-2-methylpropanesulfonic acid (AMPS)/maleic anhydride (MA) [poly(AMPS-co-MA)]. To facilitate the separation and regeneration of the adsorbent, magnetic Fe3O4 nanoparticles were embedded in the adsorbent matrix. The FT-IR, FE-SEM, EDS, XRD, VSM, and TGA were used to characterize the adsorbent. The adsorption behavior of metal ions such as Pb(II), Cu(II) and Co(II) and dyes such as methylene blue (MB) and crystal violet (CV), including the adsorption kinetics, isotherms, thermodynamics, mechanisms and the factors potentially affecting the adsorption process were investigated. Reusability of the adsorbent was evaluated by consecutive adsorption/desorption experiments.
2. Experimental
2.1. Materials
Natural graphite powder (purity 99.5%), (3-aminopropyl)triethoxysilane (APTS), 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPS) (99.0%), maleic anhydride (MA) (≥99.0%), benzoyl peroxide (BPO), methylene blue (MB), crystal violet (CV), sulfuric acid (H2SO4, 99.99%), phosphoric acid (H3PO4, ≥98.0%), potassium permanganate (KMnO4, ≥99.0%), and hydrogen peroxide solution (30% (w/w) in H2O) were purchased from Aldrich chemical company (Germany). Lead nitrate (Pb(NO3)2), cobalt nitrate (Co(NO3)2·6H2O), copper sulfate (Cu(SO4)2·5H2O), ferric chloride (FeCl3·6H2O), and ferrous chloride (FeCl2·4H2O) were purchased from Fluka (Germany). All chemicals were analytical grade and used without further purification.
2.2. Grafting of APTS onto GO nanosheets
Graphene oxide (GO) was synthesized using the improved method reported in the literature.28 In order to functionalize GO nanosheets with the amine groups, the surface of GO nanosheets was grafted on by silane coupling agent (APTS).16 0.1 g GO was dispersed in 20 mL dry toluene by ultra-sonication for 30 min. A solution of 0.2 g APTS in 5.0 mL dry chloroform was added to the GO suspension in toluene. The mixture was stirred for 72 h at 100 °C. For isolation of modified GO nanosheets, the reaction mixture was centrifuged at 4000 rpm and the obtained solid was washed with dry chloroform three times; then it was dried under vacuum at 50 °C for 24 h.
2.3. Preparation of Fe3O4 nanoparticles
Magnetic Fe3O4 nanoparticles were synthesized through conventional co-precipitation method reported in literature.29
2.4. Synthesis of poly(AMPS-co-MA)
The radical copolymerization of MA and AMPS was performed using BPO as radical initiator. Briefly, 1.0 g MA and 2.0 g AMPS were dissolved in THF, degassed for 20 min with nitrogen, then 0.3 g BPO was added and stirred at 80 °C for 12 h. The obtained poly(AMPS-co-MA) was isolated from the reaction mixture by precipitation in diethyl ether and then dried under vacuum at 50 °C.
2.5. Synthesis of magnetic adsorbent GO-APTS-poly(AMPS-co-MA)/Fe3O4
Magnetic nanocomposite adsorbent was synthesized in one-step by mixing a weight ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12 of poly(AMPS-co-MA)
0.12 of poly(AMPS-co-MA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GO-APTS
GO-APTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4 in 30 mL THF in the presence of triethylamine as a catalyst. The mixture was sonicated for 30 min and then refluxed for 12 h with stirring continuously. The nanocomposite adsorbent was collected by using a magnetic field, washed several times with deionized water, and then dried under vacuum at 50 °C for 24 h.
Fe3O4 in 30 mL THF in the presence of triethylamine as a catalyst. The mixture was sonicated for 30 min and then refluxed for 12 h with stirring continuously. The nanocomposite adsorbent was collected by using a magnetic field, washed several times with deionized water, and then dried under vacuum at 50 °C for 24 h.
2.6. Characterization methods
Fourier transform infrared spectrum (FT-IR) measurements were carried out by using Bruker vector 22 Spectrometer (Bruker, Karlsruhe, Germany) in KBr pellet at room temperature. The surface morphology of the synthesized GO and GO-based magnetic nanocomposite was examined by using field emission scanning electron microscopy (FE-SEM, HITACHI S-4160) equipped with EDS accessory. The X-ray diffraction (XRD) patterns were recorded by using X-ray diffractometer (XRD, GBC MMA Instrument) with the Cu-Kα source (λ = 1.5418 Å) at 35.4 kV and 28 mA in the 2θ region from 5° to 80°. A vibrating sample magnetometer (VSM) (Kavir Magnetic Co. Iran) was used to characterize the magnetic properties of Fe3O4 nanoparticles and magnetic nanocomposite adsorbent with an applied field from −10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to +10
000 to +10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe. In order to study the thermal behavior of the samples, thermogravimetric analysis (TGA) was performed on TGA STA 504 instrument in the temperature range of 25–600 °C under normal air with a heating rate of 10 °C min−1. A PHS-3C pH-meter (Shanghai, Tianyou) was used for pH adjustment. Flame atomic absorption instrument (nov AA 400p, Germany) (AAS) was used for measuring the concentration of metal ions in the solution. The concentration of dyes in the solution was measured by using an ultraviolet-visible (UV-vis Braic 2100) spectrophotometer.
000 Oe. In order to study the thermal behavior of the samples, thermogravimetric analysis (TGA) was performed on TGA STA 504 instrument in the temperature range of 25–600 °C under normal air with a heating rate of 10 °C min−1. A PHS-3C pH-meter (Shanghai, Tianyou) was used for pH adjustment. Flame atomic absorption instrument (nov AA 400p, Germany) (AAS) was used for measuring the concentration of metal ions in the solution. The concentration of dyes in the solution was measured by using an ultraviolet-visible (UV-vis Braic 2100) spectrophotometer.
2.7. Adsorption studies
Batch experiments were performed to examine the chelating adsorption property of GO-APTS-poly(AMPS-co-MA)/Fe3O4 adsorbent toward Pb(II), Cu(II), Co(II), MB and CV in water. The effects of main factors such as pH (2.0–8.0), adsorbent dosage (5–30 mg), contact time (1–12 h), initial metal ions and dyes concentration (20–200 mg L−1) and temperature (25–55 °C) on sorption efficiency were evaluated to determine the maximum adsorption capacity and mechanism of the adsorption. To determine the optimum dosage of adsorbent, the batch experiments were carried out using various amounts of adsorbent in 50 mL solutions of metal ions or dyes with the initial concentrations of 20 mg L−1, and then the flasks were shaken at 25 °C for a specific time. The desired pH values of solutions for metal ions (2.0–6.0) and for dyes (2.0–8.0) were adjusted with 0.1 M NaOH or 0.1 M HCl. Adsorption kinetics was studied at different time intervals at optimum pH value and constant concentration of metal ions or dyes (20 mg L−1), adsorbent dosage and temperature. The isotherm studies were also conducted with various concentrations of metal ions and dyes at optimum pH value and constant adsorbent dosage, temperature, and time. The effect of temperature on sorption was studied in 50 mL solutions of heavy metal ions or dyes (40 mg L−1). After the equilibrium, the two phases were separated and the concentration of metal ions was analyzed by using AAS. UV-vis spectrophotometer was used for determining dyes concentration via measuring the maximum absorption intensity at corresponding wavelength (λmax = 590 nm for CV and λmax = 664 nm for MB) and using following Beer–Lambert law (eqn (1)):where A is the absorption of dye at a given wavelength, ε molar absorptivity, b the path length through the solution that the light has to travel (1 cm) and C the concentration of dye in the solution. The experiments were performed in replicates of three, the average results were reported and the error bars were shown in the graphs. The following equations were used for calculating the removal percentage and adsorption capacity (Qe, mg g−1) of metal ions and dyes:| |
 | (2) |
| |
 | (3) |
where C0 and Ce (mg L−1) are the concentrations of metal ions or dyes in the initial solution and after adsorption, respectively, V is the volume of the aqueous solution (L) and m is the weight of the adsorbent (g).
2.8. Desorption and reusability of the adsorbent
Consecutive adsorption/desorption experiments were performed to assess the reusability of adsorbent. Typically, a certain amount of adsorbent (20 mg) was first saturated with metal ions and dyes. Then the adsorbent was separated from the solution by a magnet, washed with distilled water, and subsequently immersed in desorption medium. Desorption of metal ions, MB, and CV was carried out in 50 mL 0.1 M HNO3 solution, acidic methanol, and ethanol, respectively. The adsorption–desorption cycles were repeated four times. The metal ions and dyes concentrations were measured by AAS and UV-vis spectrophotometer for each adsorption cycle.
In order to determine the probable leaching of Fe ions during desorption cycle, 20 mg adsorbent was immersed into 50 mL solution of 0.1 M HNO3 and the amount of released Fe ions was measured by AAS after 24, 48, and 72 h.
3. Results and discussion
3.1. Characterization of magnetic adsorbent
The synthesis steps of magnetic adsorbent GO-APTS-poly(AMPS-co-MA)/Fe3O4 are illustrated in Schemes 1 and 2. GO was synthesized from graphite via the improved method,28 and then functionalized with amine groups by APTS grafting reaction. Poly(AMPS-co-MA) was synthesized through radical polymerization of AMPS and MA in the presence of BPO as an initiator. In the next step, poly(AMPS-co-MA) was chemically attached to GO-APTS nanosheets via ring opening reaction of maleic anhydride with the amine groups of GO-APTS. There has been an increasing attention toward separation process applying magnetic nano-sized particles.21,30 The GO-based magnetic nanocomposite GO-APTS-poly(AMPS-co-MA)/Fe3O4 was achieved by incorporating Fe3O4 nanoparticles into the GO-APTS-poly(AMPS-co-MA) matrix through physical adsorption or electrostatic interaction.
 |
| | Scheme 1 Schematic illustration of GO and GO-APTS preparation. | |
 |
| | Scheme 2 Preparation of poly(AMPS-co-MA) and GO-APTS-poly(AMPS-co-MA)/Fe3O4. | |
Fig. 1a–e shows the FT-IR spectra of Fe3O4, GO, GO-APTS, poly(AMPS-co-MA) and GO-APTS-poly(AMPS-co-MA)/Fe3O4. In the spectrum of Fe3O4 (Fig. 1a), the characteristic absorption band of the Fe–O vibration appeared at 582 cm−1. The O–H stretching vibration around 3420 cm−1 and the O–H deformed vibration at 1633 cm−1 suggest that the surface of the nanoparticles is covered with OH groups.31 In the spectrum of GO (Fig. 1b), several characteristic absorption bands are observed; for examples, O–H (3428 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1710 and 1400 cm−1), C
O (1710 and 1400 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1629 cm−1), C–OH (1229 cm−1) and C–O–C (1021 cm−1). The hydroxyl groups in the GO structure are the major anchoring sites for APTS.
C (1629 cm−1), C–OH (1229 cm−1) and C–O–C (1021 cm−1). The hydroxyl groups in the GO structure are the major anchoring sites for APTS.
 |
| | Fig. 1 FT-IR spectra of (a) Fe3O4, (b) GO, (c) GO-APTS, (d) poly(AMPS-co-MA), and (e) GO-APTS-poly(AMPS-co-MA)/Fe3O4. | |
In the spectrum of GO-APTS (Fig. 1c), the new absorption bands at 3418 and 1567 cm−1 can be assigned to the presence of NH2 groups and the absorption band at 1119 cm−1 corresponds to the vibrations of Si–O–C/Si–O–Si linkages in APTS. Also, the absorption bands at 2927 and 2862 cm−1 correspond to the methene and methyl groups of APTS. These observations show the successful covalent grafting of APTS onto GO nanosheets.16 The FT-IR spectrum of poly(AMPS-co-MA) in Fig. 1d revealed the absorption bands at 1710, 1660, and 1220 cm−1 due to the stretching vibrations of asymmetric/symmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of anhydride ring and cyclic C–O–C bonds of MA. The band at 628 cm−1 corresponds to C–S stretching of AMPS and the S
O of anhydride ring and cyclic C–O–C bonds of MA. The band at 628 cm−1 corresponds to C–S stretching of AMPS and the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in sulfonic acid appeared at 1041 cm−1.32 In the FT-IR spectrum of GO-APTS-poly(AMPS-co-MA)/Fe3O4 (Fig. 1e), the characteristic absorption bands of the anhydride ring disappeared and the characteristic absorption bands were observed at 2926 cm−1 (C–H stretching), 1636 cm−1 (C
O groups in sulfonic acid appeared at 1041 cm−1.32 In the FT-IR spectrum of GO-APTS-poly(AMPS-co-MA)/Fe3O4 (Fig. 1e), the characteristic absorption bands of the anhydride ring disappeared and the characteristic absorption bands were observed at 2926 cm−1 (C–H stretching), 1636 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1039 cm−1 (S
O stretching), 1039 cm−1 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1212 cm−1 (C–O stretching), 622 cm−1 (S–O stretching), and 675 cm−1 (Fe–O stretching).
O stretching), 1212 cm−1 (C–O stretching), 622 cm−1 (S–O stretching), and 675 cm−1 (Fe–O stretching).
The FE-SEM images of GO and magnetic adsorbent are presented in Fig. 2a and b, respectively. The surface morphology of GO presents sheet-like crumpled and rippled structure with a large thickness, which can be attributed to deformation upon the exfoliation and restacking processes. This is similar to what was observed by other researchers.33 The magnetic adsorbent shows a rough surface (Fig. 2b), revealing a hybrid surface of GO sheets that have been modified by poly(AMPS-co-MA) and magnetic Fe3O4 nanoparticles. The elements on the surface of magnetic adsorbent were examined by EDS analysis (Fig. 2c). Typical peaks correspond to C, O, Si, N, S, and Fe elements with the weight ratio of 30.21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49.76
49.76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.40
4.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.54
11.54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.16
3.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.93 were detected in the spectrum; these are the main elements in the GO-APTS-poly(AMPS-co-MA)/Fe3O4 structure.
0.93 were detected in the spectrum; these are the main elements in the GO-APTS-poly(AMPS-co-MA)/Fe3O4 structure.
 |
| | Fig. 2 FE-SEM images of (a) GO and (b) GO-APTS-poly(AMPS-co-MA)/Fe3O4, (c) EDS pattern of GO-APTS-poly(AMPS-co-MA)/Fe3O4. | |
Fig. 3a represents TGA curves of Fe3O4, GO, GO-APTS, and GO-APTS-poly(AMPS-co-MA). The Fe3O4 nanoparticles showed the initial weight loss of less than 2% at below 150 °C due to evaporation of the adsorbed moisture. The weight loss of ∼6% at around 400 °C can be related to the decomposition of Fe3O4 to α-Fe2O3.34 GO shows 10% weight loss near 100 °C, owing to the adsorbed moisture. The weight loss of ∼56% in the temperature range of 150–400 °C is related to the decomposition of oxygen-containing functional groups such as carboxyl and hydroxyl, and the weight loss beyond 400 °C is attributed to the pyrolysis of the carbon skeleton.16 In the TGA curve of GO-APTS, there is a slower mass loss of ∼8.0% around 150 °C which can be attributed to the removal of adsorbed moisture. The weight loss of 16.0% from 180 to 260 °C can be related to the release of oxygen-containing functional groups that did not react with APTS. Compared with the weight loss of GO below 300 °C, the weight loss of GO-APTS is much slower indicating the participation of numbers of hydroxyl groups of GO in the anchoring of APTS. Also, the GO-APTS showed weight loss above 300 °C which can be due to the decomposition of APTS and carbon skeleton.16 The GO-APTS-poly(AMPS-co-MA)/Fe3O4 composite showed smoother weight loss and higher thermal stability with 60% residue at 600 °C compared with the GO-APTS with ∼53% residue. This can confirm the presence of Fe3O4 nanoparticles in the magnetic adsorbent network. Fig. 3b shows the XRD patterns of GO, Fe3O4, and GO-APTS-poly(AMPS-co-MA)/Fe3O4. The GO demonstrates a dominant (001) diffraction peak at 2θ = 10° with a corresponding d-spacing of 8.7 Å. For Fe3O4, six characteristic peaks are observed at 2θ = 30.1°, 35.5°, 43.1°, 53.4°, 56.9°, and 62.5° correspond to (220), (311), (400), (422), (511), and (440) crystallographic planes, respectively. The characteristic signals of Fe3O4 have also been detected in GO-APTS-poly(AMPS-co-MA)/Fe3O4 pattern and the (001) reflection of GO shifts to 2θ = 11.6°, which can be attributed to the functionalization of GO surface. The crystal size of Fe3O4 in the prepared nanocomposite was estimated 22.13 nm using Debye–Scherrer equation. These observations prove that GO was successfully functionalized with APTS and poly(AMPS-co-MA), and also confirm the presence of Fe3O4 nanoparticles in the GO-APTS-poly(AMPS-co-MA) matrix does not alter the crystal structure of Fe3O4. The magnetic property of the prepared Fe3O4 and GO-APTS-poly(AMPS-co-MA)/Fe3O4 in Fig. 3c shows S-like curves with the saturation magnetization (σs) of 65 and 16 emu g−1, respectively. The smaller saturation magnetization of GO-APTS-poly(AMPS-co-MA)/Fe3O4 indicates that Fe3O4 nanoparticles were deeply covered by GO and the copolymer chains. The prepared GO-APTS-poly(AMPS-co-MA)/Fe3O4 exhibited superparamagnetic behavior as the remanence and coercivity of the particles is equal to zero. As can be seen in Fig. 3d, the magnetic strength of this GO-based magnetic adsorbent is sufficient for separation in aqueous solution using a conventional external magnet.
 |
| | Fig. 3 (a) TGA curves of GO, GO-APTS, GO-APTS-poly(AMPS-co-MA)/Fe3O4, and Fe3O4; (b) X-ray diffraction (XRD) analysis of neat GO, Fe3O4, and GO-APTS-poly(AMPS-co-MA)/Fe3O4; (c) VSM curves of Fe3O4 and GO-APTS-poly(AMPS-co-MA)/Fe3O4; and (d) photographic image of magnetic adsorbent separation by an external magnet. | |
3.2. Adsorption of heavy metal ions and dyes
3.2.1. Effect of pH. The pH of the solution plays a vital role in the adsorption performance as it affects the surface properties of the adsorbent, ionization/dissociation of the adsorbate molecule, and the interaction between the chelating adsorbent and the metal ions or dyes.35 Strongly ionizable sulfonic and carboxylic acid groups, which are present in the prepared magnetic adsorbent, can accept or donate protons in response to the changes in the environmental pH. For metal ions, the precipitation occurs simultaneously at pH higher than 6, which can lead to an inaccurate explanation of adsorption process. Therefore, the pH values ranging from 2 to 6 were investigated. As shown in Fig. 4a and b, the removal efficiency increases with increasing pH value of the medium. At low pH, the acid groups are mostly present in non-ionized form and no interaction can occur between these functional groups and the metal ions or dyes resulting less adsorption efficiency. The increase in adsorption at higher pH values could be attributed to the deprotonation of the acid groups, which can attach to the metal ions and cationic dyes through chelating or ion exchanges.36 The removal percentage of metal ions and cationic dyes reached the maximum level around pH 6 and 8, respectively. At these optimum pH values, the removal efficiency for Pb(II), Cu(II), Co(II), MB, and CV were 85.3%, 79.5%, 60.0%, 88.22, and 94.0% respectively. Thus, pH 6 and 8 has been accepted as the optimum pH values for further experiments.
 |
| | Fig. 4 Effect of pH (a and b) and adsorbent dosage (c and d) on the removal percentage of metal ions and dyes. | |
3.2.2. Effect of adsorbent dosage. Fig. 4c and d show that the removal percentage (R%) of the tested metal ions and dyes increases with increasing adsorbent dosage and reached the maximum values of 98.37%, 90.35%, 68.45%, 95.59%, and 99.48% for Pb(II), Cu(II), Co(II), MB, and CV, respectively at 0.4 g L−1 adsorbent. However, with further increase of the adsorbent dose, the removal percentage increases slightly. The initial sharp increase can be attributed to the increase in surface area of the adsorbent and availability of more adsorption sites. This can probably suggest that adsorption of metal ions and dyes occur mostly with active sites of the adsorbent. In addition, it is apparent that Co(II) has lower adsorption than other two tested metal ions under the same experiment conditions. This can probably be explained by the larger size of Pb(II) and Cu(II) ions which can be held more tightly inside the composite network in comparison with the smaller size of Co(II) ions.
3.2.3. Effect of initial metal ions and dyes concentration (isotherm studies). Removal of the tested metal ions and dyes by the magnetic GO-APTS-poly(AMPS-co-MA)/Fe3O4 adsorbent (0.2 g L−1) for the initial concentrations varying from 20 to 200 mg L−1, keeping all other parameters constant, was investigated at room temperature. As can be seen in Fig. 5a and b, the removal percentage of metal ions and dyes decreased with increasing the initial concentrations. The reduction in adsorption efficiency may be due to the fact that at the higher initial concentrations of pollutants the total existing adsorption sites in the adsorbent are confined.31 However, the adsorption capacity (Qe) increases with increasing the initial concentrations and reaches the maximum at a certain value, then it tends to level off at the higher sorbates concentrations. The maximum adsorption capacity was calculated by using eqn (3) and the results were 310.10, 282.25, 238.35, 416.06, and 440.97 mg g−1 for Pb(II), Cu(II), Co(II), MB, and CV, respectively. This indicates that the prepared magnetic adsorbent has great potential for removal of heavy metal ions and cationic dyes from polluted waters. Adsorption isotherm studies describe the effect of different initial concentrations on the adsorption capacity leading to the finding of the best equilibrium position and provide valuable information on the pathways of adsorption reactions. The Langmuir adsorption isotherm is based on the molecular adsorption being a monolayer and assumes that adsorption sites are identical and energetically equivalent:37,38| |
 | (4) |
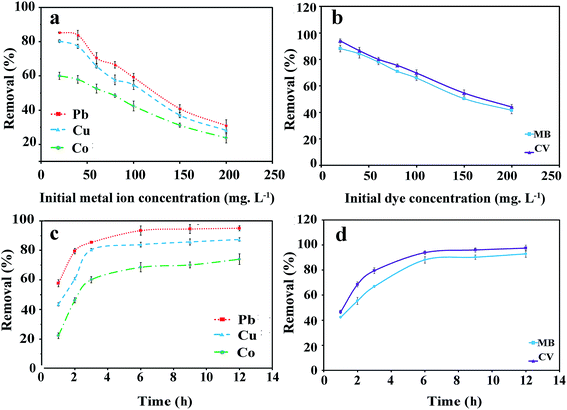 |
| | Fig. 5 Effect of the initial concentration of the tested heavy metal ions and dyes (a and b), and contact time (c and d) on the removal efficiency. | |
The Freundlich model is an empirical equation which describes the multilayer adsorption equilibrium on a heterogeneous surface:39
| |
 | (5) |
where
Qe is the equilibrium adsorption capacity (mg g
−1) and
Ce is the equilibrium sorbate concentration in solution (mg L
−1).
Qm is the maximum capacity of the adsorbent (mg g
−1),
n is the heterogeneity factor which is related to adsorption intensity, and
KF [(mg g
−1)(L mg
−1)
1/n] (related to adsorption capacity) and
KL (L mg
−1) (related to the affinity of the binding sites) are the Freundlich and Langmuir adsorption constants, which were predicted from the linear plots of ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe versus
Qe versus ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce
Ce and
 versus Ce
versus Ce, respectively.
31
According to correlation coefficients (R2) obtained from the linear form of equations and calculated isotherm parameters (Table 1), the Langmuir isotherm equation has better conformity with the experimental data than Freundlich model. The calculated maximum adsorption capacities from Langmuir model (Qm,cal) were close to the experimental values (Qm,exp). Fig. 6 shows the adsorption isotherms of the tested adsorbates at room temperature. As can be observed, the experimental data is better fitted to the Langmuir model, which confirms the monolayer adsorption of Pb(II), Cu(II), Co(II), MB, and CV by the adsorbent. As can be seen in the right corner of Fig. 6, the color of MB and CV solutions was disappeared completely after treatment with the adsorbent. The absorbance of dyes solutions decreased nearly zero indicating the high efficiency of the prepared adsorbent in the dyes removal.
Table 1 Isotherms and kinetics parameters for the adsorption of metal ions and cationic dyes onto the magnetic adsorbent at 25 °C
| Models |
Parameters |
Pb(II) |
Cu(II) |
Co(II) |
MB |
CV |
| Isotherm models |
Langmuir |
Qm (mg g−1) |
333.33 |
303.03 |
277.77 |
454.54 |
476.19 |
| Qm,exp (mg g−1) |
310.10 |
282.25 |
238.35 |
416.06 |
440.97 |
| KL (L mg−1) |
0.1388 |
0.1067 |
0.0442 |
0.0842 |
0.1055 |
| R2 |
0.9987 |
0.9981 |
0.9935 |
0.9981 |
0.9966 |
| Freundlich |
KF ((mg g−1)(L mg−1)1/n) |
79.352 |
64.566 |
29.680 |
76.853 |
96.177 |
| n |
3.157 |
2.981 |
2.189 |
2.599 |
2.855 |
| R2 |
0.8541 |
0.8696 |
0.8797 |
0.9423 |
0.9743 |
| Kinetics models |
Pseudo-first-order |
Qe,cal (mg g−1) |
49.181 |
45.593 |
50.512 |
75.840 |
71.449 |
| K1 (min−1) |
0.0087 |
0.0067 |
0.0052 |
0.0064 |
0.0076 |
| R2 |
0.9828 |
0.8971 |
0.9104 |
0.9673 |
0.9863 |
| Pseudo-second-order |
Qe,cal (mg g−1) |
100 |
95.238 |
87.719 |
106.383 |
107.526 |
| Qe,exp (mg g−1) |
94.95 |
87.30 |
73.90 |
93.018 |
97.585 |
| K2 (g mg−1 min−1) |
0.0003 |
0.0002 |
0.0001 |
0.0001 |
0.0001 |
| R2 |
0.9969 |
0.9969 |
0.9832 |
0.9974 |
0.9988 |
| Intra-particle diffusion |
kp (mg g−1 min−1/2) |
1.671 |
2.057 |
2.367 |
2.720 |
2.481 |
| C |
55.989 |
38.83 |
16.793 |
26.844 |
38.579 |
| R2 |
0.7404 |
0.7401 |
0.7917 |
0.9154 |
0.8412 |
 |
| | Fig. 6 Adsorption isotherms of metal ions and dyes; and images of MB and CV solutions before and after adsorption. | |
The GO nanosheets act as a support for polymer grafting and formation of the network. The GO nanosheets with high surface area and several chelating functional groups such as hydroxyl and carboxylic acid enhance the adsorption capacity of metal ions and dyes onto the adsorbent. In addition, the removal efficiency of CV and MB cationic dyes is increased via π–π interactions with the aromatic rings of GO.
3.2.4. Effect of contact time (kinetics studies). To determine the equilibrium time for the maximum removal efficiency, 50 mL solution of a pollutant containing 0.2 g L−1 adsorbent was stirred for various times at pH 6 and 8 for metal ions and cationic dyes, respectively. As shown in Fig. 5c and d, the removal percentages of metal ions and dyes increased rapidly initially but slowed down with further increase of the contact time. The optimum contact time was selected at 6 h. The fast adsorption at the initial contact time can be attributed to the high concentration of the adsorbates and also the abundant accessibility of the active sites on the high surface area of the adsorbent.40 Further increase in the contact time (above ∼6 h) showed a negligible effect on the removal percentage, which can be explained by the decrease in the sorbate concentration and also exhaustion of the free adsorptive sites.31 By using eqn (3), the equilibrium adsorption capacity (Qe) was determined as 94.95, 87.3, 73.9, 93.01, and 97.58 mg g−1 for Pb(II), Cu(II), Co(II), MB, and CV after 12 h, respectively. Different kinds of mechanisms such as chemical reaction and mass transfer can control the adsorption process.41 In order to study the mechanism controlling the adsorption process, three kinetic models were employed. The pseudo-first-order model is widely used for the adsorption process and is based on the assumption that the adsorption rate relates to the number of the unoccupied adsorptive sites.42 Its linearized-integral form is as follows:43| |
 | (6) |
The pseudo-second-order model predicts that the chemical sorption is a rate controlling step,42 and can be expressed by the following equation:44
| |
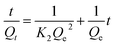 | (7) |
The intra-particle diffusion model assumes that binding of pollutant to the surface of adsorbent is affected by the mass transfer resistance,31 and the rate parameter is determined by the following equation:45
where
Qe and
Qt are the adsorption capacity (mg g
−1) at the equilibrium and at time
t, respectively, and
K1 (min
−1),
K2 (g mg
−1 min
−1), and
kp (mg g
−1 min
−1/2) are the rate constants which can be obtained from the slopes of linear plots of log(
Qe −
Qt)
versus t (
eqn (6)),
 versus t
versus t (
eqn (7)), and
Qt versus t1/2 (
eqn (8)), respectively. The kinetic models parameters and the coefficients of determination (
R2) were calculated to examine the conformity of the models with the experimental data.
Based on the results given in Table 1 and the plots in Fig. 7a–c, the values of R2 for the pseudo-second-order kinetic model are much closer to unity for the tested adsorbates, and the calculated adsorption capacities at equilibrium (Qe,cal) are in good agreement with the experimental values (Qe,exp). These results confirm the suitability of pseudo-second-order rate equation, and it is suggested that the chemical sorption involving valence forces through sharing or exchange of electrons between adsorbent and sorbate may be the rate-determining step for adsorption process.35 For the pseudo-first-order model, the correlation coefficients were found to be slightly lower and the calculated theoretical (Qe,cal) values did not agree with the experimental (Qe,exp) data. The poor fitting of the experimental data with the intra-particle diffusion model revealed that this kinetic model is not the rate-limiting step in the adsorption.
 |
| | Fig. 7 Kinetic models for the adsorption of metal ions and dyes: (a) pseudo-first-order, (b) pseudo-second-order, and (c) intra-particle diffusion model. | |
3.2.5. Adsorption thermodynamics. The adsorption of the tested metal ions and dyes onto the magnetic GO-APTS-poly(AMPS-co-MA)/Fe3O4 adsorbent was studied in the temperature range of 25–55 °C using 50 mL solutions of 40 mg L−1 pollutant, the results are listed in Table 2. According to Fig. 8a, the removal efficiency increases with increasing temperature which can be due to the increase in mobility of the sorbates molecules and increase the number of molecules with sufficient energy to interact with active sites at the surface.46 This observation indicates that the adsorption process was endothermic in nature. The values of thermodynamic parameters including enthalpy (ΔH0), entropy (ΔS0), and Gibbs free energy (ΔG0) changes were calculated using the following classical equations (eqn (9) and (10)):| |
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL KL
| (9) |
| |
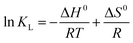 | (10) |
where R is the universal gas constant (8.314 J mol−1 K−1), T is the absolute temperature (K), and KL is the ratio of the concentration of pollutants adsorbed onto the magnetic adsorbent to their remaining concentration in solution at equilibrium (Ce). Considering the eqn (10), ΔH0 and ΔS0 can be calculated from the slope and intercept of the plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL versus 1/T (Fig. 8b). The negative values of ΔG0 (Table 2) suggest that the adsorption process is thermodynamically feasible and spontaneous at the room temperature. Furthermore, the decrease in ΔG0 values with increasing temperature demonstrates that degree of spontaneity and feasibility of adsorption increases at higher temperature. The adsorption enthalpy changes (ΔH0) were calculated to be 26.315, 27.466, 23.087, 63.012, and 76.867 kJ mol−1 for Pb(II), Cu(II), Co(II), MB, and CV, respectively, which are in the ΔH0 range for chemisorption (20.9–418.4 kJ mol−1) with positive values.47 Moreover, positive values of ΔS0 display an irregular increase of the randomness at the magnetic adsorbent–solution interface during adsorption.
KL versus 1/T (Fig. 8b). The negative values of ΔG0 (Table 2) suggest that the adsorption process is thermodynamically feasible and spontaneous at the room temperature. Furthermore, the decrease in ΔG0 values with increasing temperature demonstrates that degree of spontaneity and feasibility of adsorption increases at higher temperature. The adsorption enthalpy changes (ΔH0) were calculated to be 26.315, 27.466, 23.087, 63.012, and 76.867 kJ mol−1 for Pb(II), Cu(II), Co(II), MB, and CV, respectively, which are in the ΔH0 range for chemisorption (20.9–418.4 kJ mol−1) with positive values.47 Moreover, positive values of ΔS0 display an irregular increase of the randomness at the magnetic adsorbent–solution interface during adsorption.
Table 2 Thermodynamic parameters for metal ions and cationic dyes adsorption
| Metal ions and dyes |
ΔH0 (kJ mol−1) |
ΔS0 (J mol−1 K−1) |
−ΔG0 (kJ mol−1) |
| 25 °C |
35 °C |
45 °C |
55 °C |
| Pb(II) |
26.315 |
114.891 |
8.059 |
8.927 |
10.056 |
11.538 |
| Cu(II) |
27.466 |
115.306 |
7.016 |
7.939 |
9.010 |
10.527 |
| Co(II) |
23.087 |
93.4999 |
4.779 |
5.709 |
6.635 |
7.588 |
| MB |
63.012 |
237.314 |
8.149 |
9.590 |
11.989 |
15.341 |
| CV |
76.867 |
284.737 |
8.611 |
10.367 |
12.515 |
17.523 |
 |
| | Fig. 8 (a) Effect of temperature on the tested metal ions and dyes removal and (b) adsorption thermodynamics of the tested metal ions and dyes. | |
3.3. Desorption and regeneration studies
It is necessary to examine the possibility of recovering adsorbed pollutants and recycling the adsorbent for environmental and economic reasons. To determine the reusability of the magnetic adsorbent, consecutive adsorption–desorption cycles were repeated four times under the same conditions. The removal efficiency was calculated for each cycle by using eqn (2). The results in Fig. 9 show that no significant decrease in the removal percentage was observed after four cycles of adsorption/desorption. The recyclability is an important factor for evaluating the economy and applicability of adsorbents. The results indicate that the magnetic GO-APTS-poly(AMPS-co-MA) could be a cost-effective and an efficient adsorbent for metal ions and cationic dyes due to the excellent regeneration and reusability performance. The leaching of Fe ions from this magnetic adsorbent after several recycling cycles under acidic condition was examined. The amount of released Fe ions into HNO3 solution was only 2.231, 3.542, and 3.978 mg L−1 after 24, 48, and 72 h leaching, respectively.
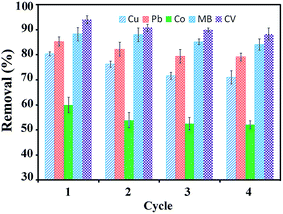 |
| | Fig. 9 Reusability of GO-APTS-poly(AMPS-co-MA) magnetic adsorbent for metal ions and dyes removal during four cycles. | |
The comparison of the maximum adsorption capacity of the present magnetic adsorbent with the other adsorbents previously reported for the adsorption of metal ions and cationic dyes is listed in Table 3. As can be seen, the adsorption capacity of the prepared adsorbent composite is in the range of 238–310 mg g−1 for tested metal ions and above 416 mg g−1 for the cationic dyes at 25 °C. These values are much higher than adsorption values for other adsorbents such as porous GO/CMC monoliths,48 GO sponge,49 graphene oxide,50 magnetic chitosan/graphene oxide,51 and for the adsorption of MB is almost comparable with RL-GO.20
Table 3 Maximum adsorption capacities for the metal ions and cationic dyes onto various adsorbents
| Adsorbent |
Qm (mg g−1) |
Ref. |
| Pb(II) |
Cu(II) |
Co(II) |
MB |
CV |
| Porous GO/CMC monoliths |
76.70 |
82.93 |
59.99 |
— |
— |
48 |
| GO sponge |
— |
— |
— |
389.84 |
— |
49 |
| GO |
— |
— |
— |
240.65 |
— |
50 |
| MCGO |
— |
— |
— |
95.16 |
— |
51 |
| MCGO |
76.94 |
— |
— |
— |
— |
52 |
| Graphene nanosheets (GNSs) |
35.7 |
— |
— |
— |
— |
53 |
| PAH functionalized GO |
— |
349.03 |
— |
— |
— |
54 |
| Sulfonated magnetic GO |
— |
62.73 |
— |
— |
— |
55 |
| Magnetite/graphene oxide (M/GO) |
— |
— |
12.98 |
— |
— |
56 |
| TiO2–graphene hydrogel |
— |
— |
— |
120 |
— |
57 |
| Magnetic graphene oxide |
— |
— |
— |
64.23 |
— |
58 |
| Fe3O4@APS@AA-co-CA MNPs |
— |
— |
— |
142.9 |
208.3 |
59 |
| Magnetic Fe3O4@SiO2 starch-graft-poly(acrylic acid) |
— |
— |
— |
— |
80.64 |
23 |
| MWCNTs/ferrite magnetic nanocomposite |
— |
— |
— |
— |
5.00 |
41 |
| Rhamnolipid-functionalized graphene oxide (RL-GO) |
— |
— |
— |
529.10 |
— |
20 |
| GO-APTS-poly(AMPS-co-MA)/magnetic nanocomposite |
310.10 |
282.25 |
238.35 |
416.06 |
440.97 |
This work |
The adsorption process of heavy metal ions and dyes often includes a complex adsorption mechanism and various types of interactions. The high performance of the GO-APTS-poly(AMPS-co-MA)/Fe3O4 adsorbent toward positively charged adsorbates may be ascribed to the physical adsorption, chemical adsorption, and electrostatic interaction. The adsorption process may involve ion–exchange reaction and surface complexation between metal cations and several chelating groups of the adsorbent such as –COOH, –OH, –SO3H, and –NHCO. For cationic dyes, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds and benzene rings with π electrons of CV and MB could form the π–π bond with the aromatic rings of GO nanosheets. Therefore, the adsorption mechanism of cationic dyes is proposed to be chemical adsorption through the strong π–π stacking and anion–cation interaction, and the adsorption of metal ions can take place at the functional groups or binding sites on the surface of adsorbents in a monolayer manner.20,60
C double bonds and benzene rings with π electrons of CV and MB could form the π–π bond with the aromatic rings of GO nanosheets. Therefore, the adsorption mechanism of cationic dyes is proposed to be chemical adsorption through the strong π–π stacking and anion–cation interaction, and the adsorption of metal ions can take place at the functional groups or binding sites on the surface of adsorbents in a monolayer manner.20,60
4. Conclusions
The objective of this work was the preparation of a novel magnetic nanocomposite adsorbent [GO-APTS-poly(AMPS-co-MA)/Fe3O4] with super adsorption capacity for the removal of metal ions and cationic dyes from water. The prepared magnetic adsorbent is rich of oxygen-containing functional groups such as carboxylic and sulfonic acid groups with negatively charged surface in the pH range higher than 4. The magnetic adsorbent was fully characterized by FT-IR, FE-SEM, EDS, XRD, and TGA, and the adsorption process of Pb(II), Cu(II), Co(II), MB, and CV showed dependence on the initial sorbates concentration, adsorbent dosage, contact time, initial solution pH and temperature. The adsorption followed the pseudo-second-order kinetic model, agreed well with Langmuir isothermal model, and was found to increase with increasing temperature. The adsorption process was favorable and spontaneous with increased disorder and randomness at the interface of solid-solution, and endothermic of physical and chemical sorption processes due to the electrostatic attraction and hydrogen bonding between the magnetic adsorbent and sorbates. The maximum adsorption capacity for Pb(II), Cu(II), Co(II), MB, and CV were calculated as 310.10, 282.25, 238.25, 416.06, and 440.97 mg g−1, respectively. These features show that the prepared magnetic nanocomposite can be an efficient and potential adsorbent due to its excellent reusability, facile collection through a magnetic field, cost-effective, and outstanding performance in removal of metal ions and cationic dyes from water.
References
- A. Duran, M. Soylak and S. A. Tuncel, J. Hazard. Mater., 2008, 155, 114–120 CrossRef CAS PubMed
 .
. - A. Idris, N. S. M. Ismail, N. Hassan, E. Misran and A.-F. Ngomsik, J. Ind. Eng. Chem., 2012, 18, 1582–1589 CrossRef CAS
 .
. - M. Kumar, B. P. Tripathi and V. K. Shahi, J. Hazard. Mater., 2009, 172, 1041–1048 CrossRef CAS PubMed
 .
. - M. M. Matlock, B. S. Howerton and D. A. Atwood, Water Res., 2002, 36, 4757–4764 CrossRef CAS PubMed
 .
. - I. Heidmann and W. Calmano, J. Hazard. Mater., 2008, 152, 934–941 CrossRef CAS PubMed
 .
. - M. K. Doula, Water Res., 2009, 43, 3659–3672 CrossRef CAS PubMed
 .
. - H. Bessbousse, T. Rhlalou, J.-F. Verchère and L. Lebrun, J. Membr. Sci., 2008, 307, 249–259 CrossRef CAS
 .
. - M. Sprynskyy, J. Hazard. Mater., 2009, 161, 1377–1383 CrossRef CAS PubMed
 .
. - H. Ozaki, K. Sharma and W. Saktaywin, Desalination, 2002, 144, 287–294 CrossRef CAS
 .
. - X. Li, Y. Qi, Y. Li, Y. Zhang, X. He and Y. Wang, Bioresour. Technol., 2013, 142, 611–619 CrossRef CAS PubMed
 .
. - H. Gao, Y. Sun, J. Zhou, R. Xu and H. Duan, ACS Appl. Mater. Interfaces, 2013, 5, 425–432 CAS
 .
. - Y. Wan, X. Chen, G. Xiong, R. Guo and H. Luo, Mater. Express, 2014, 4, 429–434 CrossRef CAS
 .
. - L. Fan, C. Luo, M. Sun, X. Li, F. Lu and H. Qiu, Bioresour. Technol., 2012, 114, 703–706 CrossRef CAS PubMed
 .
. - R. Xing, T. Jiao, Y. Liu, K. Ma, Q. Zou, G. Ma and X. Yan, Polymers, 2016, 8, 181 CrossRef
 .
. - Y. Huang, M. Zeng, J. Ren, J. Wang, L. Fan and Q. Xu, Colloids Surf., A, 2012, 401, 97–106 CrossRef CAS
 .
. - J. Huang, S. Ding, W. Xiao, Y. Peng, S. Deng and N. Zhang, Catal. Lett., 2015, 145, 1000–1007 CrossRef CAS
 .
. - Y. Zhou, M. Zhang, X. Wang, Q. Huang, Y. Min, T. Ma and J. Niu, Ind. Eng. Chem. Res., 2014, 53, 5498–5506 CrossRef CAS
 .
. - Y. Zhou, M. Zhang, X. Hu, X. Wang, J. Niu and T. Ma, J. Chem. Eng. Data, 2013, 58, 413–421 CrossRef CAS
 .
. - F. Zhang, B. Wang, S. He and R. Man, J. Chem. Eng. Data, 2014, 59, 1719–1726 CrossRef CAS
 .
. - Z. Wu, H. Zhong, X. Yuan, H. Wang, L. Wang, X. Chen, G. Zeng and Y. Wu, Water Res., 2014, 67, 330–344 CrossRef CAS PubMed
 .
. - A. Masoumi, M. Ghaemy and A. N. Bakht, Ind. Eng. Chem. Res., 2014, 53, 8188–8197 CrossRef CAS
 .
. - V. Gupta, S. Agarwal and T. A. Saleh, Water Res., 2011, 45, 2207–2212 CrossRef CAS PubMed
 .
. - A. Pourjavadi, S. H. Hosseini, F. Seidi and R. Soleyman, Polym. Int., 2013, 62, 1038–1044 CrossRef CAS
 .
. - G. Xie, P. Xi, H. Liu, F. Chen, L. Huang, Y. Shi, F. Hou, Z. Zeng, C. Shao and J. Wang, J. Mater. Chem., 2012, 22, 1033–1039 RSC
 .
. - T. Jiao, Y. Liu, Y. Wu, Q. Zhang, X. Yan, F. Gao, A. J. Bauer, J. Liu, T. Zeng and B. Li, Sci. Rep., 2015, 5, 12451 CrossRef CAS PubMed
 .
. - H. Guo, T. Jiao, Q. Zhang, W. Guo, Q. Peng and X. Yan, Nanoscale Res. Lett., 2015, 10, 1–10 CrossRef CAS PubMed
 .
. - W. Wang, T. Jiao, Q. Zhang, X. Luo, J. Hu, Y. Chen, Q. Peng, X. Yan and B. Li, RSC Adv., 2015, 5, 56279–56285 RSC
 .
. - D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed
 .
. - X. Liu, Z. Ma, J. Xing and H. Liu, J. Magn. Magn. Mater., 2004, 270, 1–6 CrossRef CAS
 .
. - S. Yang, P. Zong, X. Ren, Q. Wang and X. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 6891–6900 CAS
 .
. - Z. S. Pour and M. Ghaemy, RSC Adv., 2015, 5, 64106–64118 RSC
 .
. - Y. G. Devrim, Z. M. Rzaev and E. Pişkin, Macromol. Chem. Phys., 2006, 207, 111–121 CrossRef CAS
 .
. - C. Fu, G. Zhao, H. Zhang and S. Li, Int. J. Electrochem. Sci., 2013, 8, 6269–6280 CAS
 .
. - O. Philippova, A. Barabanova, V. Molchanov and A. Khokhlov, Eur. Polym. J., 2011, 47, 542–559 CrossRef CAS
 .
. - A. Masoumi, K. Hemmati and M. Ghaemy, RSC Adv., 2015, 5, 1735–1744 RSC
 .
. - H. Kaşgöz, S. Özgümüş and M. Orbay, Polymer, 2003, 44, 1785–1793 CrossRef
 .
. - I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS
 .
. - A. Masoumi, K. Hemmati and M. Ghaemy, Chemosphere, 2016, 146, 253–262 CrossRef CAS PubMed
 .
. - H. Freundlich, Trans. Faraday Soc., 1932, 28, 195–201 RSC
 .
. - E. Ramírez, S. G. Burillo, C. Barrera-Díaz, G. Roa and B. Bilyeu, J. Hazard. Mater., 2011, 192, 432–439 CrossRef PubMed
 .
. - M. Gabal, E. Al-Harthy, Y. Al Angari and M. A. Salam, Chem. Eng. J., 2014, 255, 156–164 CrossRef CAS
 .
. - A. Masoumi and M. Ghaemy, Carbohydr. Polym., 2014, 108, 206–215 CrossRef CAS PubMed
 .
. - S. Lagergren, K. Sven. Vetenskapsakad. Handl., 1898, 24, 1–39 Search PubMed
 .
. - Y. Önal, C. Akmil-Başar, D. Eren, Ç. Sarıcı-Özdemir and T. Depci, J. Hazard. Mater., 2006, 128, 150–157 CrossRef PubMed
 .
. - X. Mi, G. Huang, W. Xie, W. Wang, Y. Liu and J. Gao, Carbon, 2012, 50, 4856–4864 CrossRef CAS
 .
. - L. Wang, J. Zhang and A. Wang, Colloids Surf., A, 2008, 322, 47–53 CrossRef CAS
 .
. - L. Wang and A. Wang, J. Hazard. Mater., 2007, 147, 979–985 CrossRef CAS PubMed
 .
. - Y. Zhang, Y. Liu, X. Wang, Z. Sun, J. Ma, T. Wu, F. Xing and J. Gao, Carbohydr. Polym., 2014, 101, 392–400 CrossRef CAS PubMed
 .
. - F. Liu, S. Chung, G. Oh and T. S. Seo, ACS Appl. Mater. Interfaces, 2012, 4, 922–927 CAS
 .
. - Y. Li, Q. Du, T. Liu, X. Peng, J. Wang, J. Sun, Y. Wang, S. Wu, Z. Wang and Y. Xia, Chem. Eng. Res. Des., 2013, 91, 361–368 CrossRef CAS
 .
. - L. Fan, C. Luo, X. Li, F. Lu, H. Qiu and M. Sun, J. Hazard. Mater., 2012, 215, 272–279 CrossRef PubMed
 .
. - L. Fan, C. Luo, M. Sun, X. Li and H. Qiu, Colloids Surf., B, 2013, 103, 523–529 CrossRef CAS PubMed
 .
. - Z.-H. Huang, X. Zheng, W. Lv, M. Wang, Q.-H. Yang and F. Kang, Langmuir, 2011, 27, 7558–7562 CrossRef CAS PubMed
 .
. - H. T. Xing, J. H. Chen, X. Sun, Y. H. Huang, Z. B. Su, S. R. Hu, W. Weng, S. X. Li, H. X. Guo and W. B. Wu, Chem. Eng. J., 2015, 263, 280–289 CrossRef CAS
 .
. - X.-j. Hu, Y.-g. Liu, H. Wang, A.-w. Chen, G.-m. Zeng, S.-m. Liu, Y.-m. Guo, X. Hu, T.-t. Li and Y.-q. Wang, Sep. Purif. Technol., 2013, 108, 189–195 CrossRef CAS
 .
. - M. Liu, C. Chen, J. Hu, X. Wu and X. Wang, J. Phys. Chem. C, 2011, 115, 25234–25240 CAS
 .
. - Z. Zhang, F. Xiao, Y. Guo, S. Wang and Y. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 2227–2233 CAS
 .
. - J.-H. Deng, X.-R. Zhang, G.-M. Zeng, J.-L. Gong, Q.-Y. Niu and J. Liang, Chem. Eng. J., 2013, 226, 189–200 CrossRef CAS
 .
. - F. Ge, H. Ye, M.-M. Li and B.-X. Zhao, Chem. Eng. J., 2012, 198, 11–17 CrossRef
 .
. - C. J. Madadrang, H. Y. Kim, G. Gao, N. Wang, J. Zhu, H. Feng, M. Gorring, M. L. Kasner and S. Hou, ACS Appl. Mater. Interfaces, 2012, 4, 1186–1193 CAS
 .
.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12 of poly(AMPS-co-MA)
0.12 of poly(AMPS-co-MA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GO-APTS
GO-APTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4 in 30 mL THF in the presence of triethylamine as a catalyst. The mixture was sonicated for 30 min and then refluxed for 12 h with stirring continuously. The nanocomposite adsorbent was collected by using a magnetic field, washed several times with deionized water, and then dried under vacuum at 50 °C for 24 h.
Fe3O4 in 30 mL THF in the presence of triethylamine as a catalyst. The mixture was sonicated for 30 min and then refluxed for 12 h with stirring continuously. The nanocomposite adsorbent was collected by using a magnetic field, washed several times with deionized water, and then dried under vacuum at 50 °C for 24 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to +10
000 to +10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe. In order to study the thermal behavior of the samples, thermogravimetric analysis (TGA) was performed on TGA STA 504 instrument in the temperature range of 25–600 °C under normal air with a heating rate of 10 °C min−1. A PHS-3C pH-meter (Shanghai, Tianyou) was used for pH adjustment. Flame atomic absorption instrument (nov AA 400p, Germany) (AAS) was used for measuring the concentration of metal ions in the solution. The concentration of dyes in the solution was measured by using an ultraviolet-visible (UV-vis Braic 2100) spectrophotometer.
000 Oe. In order to study the thermal behavior of the samples, thermogravimetric analysis (TGA) was performed on TGA STA 504 instrument in the temperature range of 25–600 °C under normal air with a heating rate of 10 °C min−1. A PHS-3C pH-meter (Shanghai, Tianyou) was used for pH adjustment. Flame atomic absorption instrument (nov AA 400p, Germany) (AAS) was used for measuring the concentration of metal ions in the solution. The concentration of dyes in the solution was measured by using an ultraviolet-visible (UV-vis Braic 2100) spectrophotometer.


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1710 and 1400 cm−1), C
O (1710 and 1400 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1629 cm−1), C–OH (1229 cm−1) and C–O–C (1021 cm−1). The hydroxyl groups in the GO structure are the major anchoring sites for APTS.
C (1629 cm−1), C–OH (1229 cm−1) and C–O–C (1021 cm−1). The hydroxyl groups in the GO structure are the major anchoring sites for APTS.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of anhydride ring and cyclic C–O–C bonds of MA. The band at 628 cm−1 corresponds to C–S stretching of AMPS and the S
O of anhydride ring and cyclic C–O–C bonds of MA. The band at 628 cm−1 corresponds to C–S stretching of AMPS and the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in sulfonic acid appeared at 1041 cm−1.32 In the FT-IR spectrum of GO-APTS-poly(AMPS-co-MA)/Fe3O4 (Fig. 1e), the characteristic absorption bands of the anhydride ring disappeared and the characteristic absorption bands were observed at 2926 cm−1 (C–H stretching), 1636 cm−1 (C
O groups in sulfonic acid appeared at 1041 cm−1.32 In the FT-IR spectrum of GO-APTS-poly(AMPS-co-MA)/Fe3O4 (Fig. 1e), the characteristic absorption bands of the anhydride ring disappeared and the characteristic absorption bands were observed at 2926 cm−1 (C–H stretching), 1636 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1039 cm−1 (S
O stretching), 1039 cm−1 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1212 cm−1 (C–O stretching), 622 cm−1 (S–O stretching), and 675 cm−1 (Fe–O stretching).
O stretching), 1212 cm−1 (C–O stretching), 622 cm−1 (S–O stretching), and 675 cm−1 (Fe–O stretching).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49.76
49.76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.40
4.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.54
11.54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.16
3.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.93 were detected in the spectrum; these are the main elements in the GO-APTS-poly(AMPS-co-MA)/Fe3O4 structure.
0.93 were detected in the spectrum; these are the main elements in the GO-APTS-poly(AMPS-co-MA)/Fe3O4 structure.



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe versus ln
Qe versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce and
Ce and  versus Ce, respectively.31
versus Ce, respectively.31


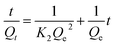
 versus t (eqn (7)), and Qt versus t1/2 (eqn (8)), respectively. The kinetic models parameters and the coefficients of determination (R2) were calculated to examine the conformity of the models with the experimental data.
versus t (eqn (7)), and Qt versus t1/2 (eqn (8)), respectively. The kinetic models parameters and the coefficients of determination (R2) were calculated to examine the conformity of the models with the experimental data.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL
KL
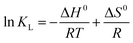
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL versus 1/T (Fig. 8b). The negative values of ΔG0 (Table 2) suggest that the adsorption process is thermodynamically feasible and spontaneous at the room temperature. Furthermore, the decrease in ΔG0 values with increasing temperature demonstrates that degree of spontaneity and feasibility of adsorption increases at higher temperature. The adsorption enthalpy changes (ΔH0) were calculated to be 26.315, 27.466, 23.087, 63.012, and 76.867 kJ mol−1 for Pb(II), Cu(II), Co(II), MB, and CV, respectively, which are in the ΔH0 range for chemisorption (20.9–418.4 kJ mol−1) with positive values.47 Moreover, positive values of ΔS0 display an irregular increase of the randomness at the magnetic adsorbent–solution interface during adsorption.
KL versus 1/T (Fig. 8b). The negative values of ΔG0 (Table 2) suggest that the adsorption process is thermodynamically feasible and spontaneous at the room temperature. Furthermore, the decrease in ΔG0 values with increasing temperature demonstrates that degree of spontaneity and feasibility of adsorption increases at higher temperature. The adsorption enthalpy changes (ΔH0) were calculated to be 26.315, 27.466, 23.087, 63.012, and 76.867 kJ mol−1 for Pb(II), Cu(II), Co(II), MB, and CV, respectively, which are in the ΔH0 range for chemisorption (20.9–418.4 kJ mol−1) with positive values.47 Moreover, positive values of ΔS0 display an irregular increase of the randomness at the magnetic adsorbent–solution interface during adsorption.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds and benzene rings with π electrons of CV and MB could form the π–π bond with the aromatic rings of GO nanosheets. Therefore, the adsorption mechanism of cationic dyes is proposed to be chemical adsorption through the strong π–π stacking and anion–cation interaction, and the adsorption of metal ions can take place at the functional groups or binding sites on the surface of adsorbents in a monolayer manner.20,60
C double bonds and benzene rings with π electrons of CV and MB could form the π–π bond with the aromatic rings of GO nanosheets. Therefore, the adsorption mechanism of cationic dyes is proposed to be chemical adsorption through the strong π–π stacking and anion–cation interaction, and the adsorption of metal ions can take place at the functional groups or binding sites on the surface of adsorbents in a monolayer manner.20,60.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.






