Influences of the Pb 6s2 lone pair effect and quantum size effect on the diffusion of oxygen atoms on Pb(111) films†
Abstract
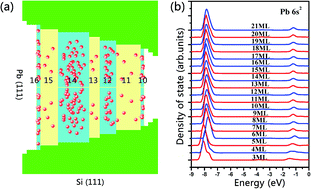
Based on our previous studies revealing quantum oscillations in the adsorption energetics of atomic oxygen on Pb(111) films, here we study all the possible on-surface and subsurface adsorption sites of oxygen atoms on Pb(111) films at different coverages. The Pb 6s2 bilayer oscillation behavior is compared with relative basic physical and chemical properties such as adsorption energy or diffusion, allowing us to conclude that the intensity of the lone pair effect of Pb 6s2 directly correlates with the bilayer oscillation amplitudes of adsorption energy and diffusion barriers. A stronger Pb 6s2 lone pair effect depresses the density of states at the Fermi level, which leads to the prominent bilayer oscillations of the adsorption energy and diffusion barriers. In contrast, a weaker Pb 6s2 lone pair effect frees the Pb 6px and 6py electrons interacting with O atoms near the Fermi level, disrupting the bilayer oscillations.

 Please wait while we load your content...
Please wait while we load your content...