High yield synthesis of amine functionalized graphene oxide and its surface properties†
Abstract
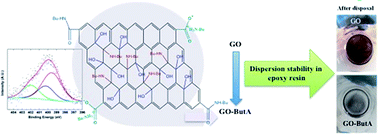
Graphene and its derivatives have attracted great research interest due to their many exciting properties leading to a number of potential applications. However, for many practical applications including reinforcement in epoxy resin matrices, chemical functionalization of graphene is often a necessary requirement. Herein we report a simple temperature-assisted reflux method to synthesize graphene oxide (GO) functionalized with n-butylamine in high yield without the use of toxic chemicals. X-ray photoelectron spectroscopy and energy dispersive analysis by X-rays showed successful attachment of amine groups onto GO to form (GO-ButA). The functionalized GO was further characterized using Raman, nuclear magnetic resonance and Fourier transform infrared spectroscopies. The surface morphology and particle size, etc. were characterized using scanning electron microscopy. The solution properties of the GO-ButA were investigated by dispersing in water and common organic solvents which indicated an increased hydrophobic nature of the product which was also confirmed by contact angle measurements. Further, the interaction of GO-ButA with epoxy resin was tested by dispersing it in an epoxy resin which indicated an improved and more stable dispersion as compared to that of GO which shows the potential application of GO-ButA as a reinforcement (filler) in epoxy based composites.

 Please wait while we load your content...
Please wait while we load your content...