Selective picomolar level fluorometric sensing of the Cr(vi)-oxoanion in a water medium by a novel metal–organic complex†
Abstract
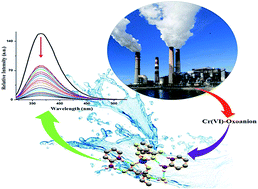
A novel metal–organic complex (MOC) of Cu(II) with n-butylmalonate ligands and protonated 2-aminopyridimium rings has been synthesized and structurally characterized by single-crystal X-ray diffraction. An intriguing supramolecular interaction i.e. hydrogen-bonding pattern (like N–H⋯O and O–H⋯O) assisted lone-pair⋯π/π⋯π assembly is observed in the crystalline form of the MOC. The role of different non-covalent contacts in the formation of the MOC in the solid-state has also been scrutinized through Hirshfeld Surface Analysis. The luminescence properties of the MOC in the water solution were also experimentally investigated. The aqueous solution of the MOC acts as a selective picomolar level fluorescent sensor for Cr(VI)-oxoanion in water medium. Even the presence of several other cations like Li+, Na+, K+, Ca2+, Mg2+, Fe2+, Co2+, Ni2+, Cd2+, Hg2+, Zn2+, another chromium source like Cr3+ and versatile anions including F−, Cl−, Br−, SO42−, N3−, NO3−, BF4−, ClO4−, AsO33−, PO43− do not interfere with the picomolar level Cr(VI)-oxoanion sensing ability of the MOC in water medium. The probable sensing mechanism of the Cr(VI)-oxoanion by the MOC has been investigated experimentally and theoretically.

 Please wait while we load your content...
Please wait while we load your content...