Soy-castor oil based polyurethanes with octaphenylsilsesquioxanetetraol double-decker silsesquioxane in the main chains
Jie Huanga,
Pingping Jiang*a,
Yue Wena,
Jianneng Dengb and
Jian Heb
aThe Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, China. E-mail: ppjiang@jiangnan.edu.cn
bNantong Hairma Technology Co., Ltd, Nantong 226000, China
First published on 6th July 2016
Abstract
An environmentally friendly, solvent-free/catalyst-free method was used to prepare soy-castor oil based polyols (SCPs) from epoxy soybean oil and castor oil. Thereafter, a series of hybrid polyurethanes using SCPs as feedstocks were synthesized with the introduction of double-decker silsesquioxane. Double-decker octaphenylsilsesquioxanetetraol (DDSQ) was prepared and characterized by 1H NMR and MALDI-TOF-MS. Meanwhile, the soy-castor oil based polyols (SCPs) were also characterized by 1H NMR and FTIR. Other characterizations such as DMA, TGA, SEM, FTIR, XRD, static contact angle and tensile test techniques were also applied to investigate the structures and properties of the hybrid polyurethanes. 1H NMR spectra indicated that DDSQ was accessed in the form of chemical bonding sealing on polyurethane pre-polymers. DMA analysis demonstrated that the hybrid polyurethanes with DDSQ displayed enhanced glass transition temperatures (Tg) compared to the pure polyurethanes. The DDSQ-containing hybrid polyurethanes exhibited improved thermal stability in terms of thermogravimetric analysis (TGA). SEM revealed that both nano- and micro-sized DDSQ aggregates were shown to be dispersed heterogeneously in the polyurethane matrix. The results of XRD showed that DDSQ formed a nanocrystalline phase in any of the PUs. With the inclusion of DDSQ, the hydrophobicity of the hybrid material was significantly improved, as the results of the static contact angles revealed.
1. Introduction
Composed of a cage like Si–O framework and several organic groups covalently bonded to each Si atom, one or more of which is reactive. Polyhedral oligomeric silsesquioxanes (POSS) have attracted much attention. Because of the unique three-dimensional structure, the external organic substituents can be replaced by a range of polar or nonpolar functional groups.1 The well-defined organometallic features and the nanostructures of POSS inspire one to incorporate this class of nanobuilding blocks into organic polymers to afford organic–inorganic hybrids with improved properties. Specifically, the synthetic routes of POSS are roughly divided into four categories, substitution reactions with retention of the siloxane cage, the hydrolysis and condensation of trifunctional silsesquioxanes, the functionalization of the preformed POSS cage and corner-capping reactions.2 However, the application of the POSS monomer is limited to some extent. In order to overcome the shortcomings, POSS derivatives possessing unique performances and various active functional groups are synthesized according to the appropriate functionalized approach to POSS.3,4 Generally, POSS derivatives depending on the number of the reactive functional groups classify into three: monofunctional POSS, difunctional POSS and multi-functional POSS. For monofunctional POSS, it is reported that POSS cages behave as either end groups or pendent side groups in the polymers and the main chains of the polymers remain unchanged. The thermal, nonflammability, mechanical properties (e.g., strength, modulus, rigidity), excellent dielectric properties, and oxidative resistance of the POSS-containing hybrid materials are enhanced.5–7 For multi-functional POSS, there have been some reports,8–14 in which octafunctional POSS macromers are mostly investigated. For instance, large amount of POSS-containing nanocomposites like polyimide–POSS copolymers,8 polyurethane–POSS copolymers,9 poly(methyl methacrylate)–POSS copolymers,10 epoxy–POSS copolymers,11 poly(ethylene imine)–POSS copolymers12 and polybenzoxazines–POSS copolymers.13,14 Among above nanocomposites, POSS cages behave as the nanosized crosslinkers to take part in the formation of polymer networks. Attributed to the presence of POSS cages in the main chains of the crosslinked polymers, the properties of these nanocomposites obtained a great improvement. Nonetheless, for many thermoplastic materials, suitable difunctional POSS is introduced into the main chains of polymers via a step-growth polymerization approach. There have been a few reports on the synthesis and characterization of the difunctional POSS. Kakimoto et al.15 reported the preparation and characterization of hybrid polyimides with double-decker silsesquioxane (DDSQ) in the main chains, and drew a conclusion that the hybrid polyimides exhibited improved dielectric and thermomechanical properties with the DDSQ in the main chain. More recently, Kawakami et al.16 reported the synthesis of the polysiloxanes–DDSQ copolymers, the reaction of cross-dehydrocoupling and polycondensation was between DDSQ and octamethyltetrasiloxane. In previous work, Huang and Jiang17 reported the synthesis and characterization of sustainable polyurethane based on epoxy soybean oil and modified by double-decker silsesquioxane (DDSQ), it was found that the thermomechanical properties, the glass transition temperatures (Tg) and the hydrophobicity were improved with the DDSQ in the main chain.Polyurethanes (PUs) are significant class of materials which exhibit various applications. Selecting appropriate polyols or isocyanates, PUs can be tailored to meet a variety of specific requirements. In addition, the increasing price of crude oil and environmental concerns have triggered great attention on the development of materials which based on renewable resources. Due to their variety and economy, many vegetable oils such as soybean oil, castor oil, rapeseed and canola have been chosen as feedstock in the polyurethane industry.18 They also own excellent properties, including inherent sustainability, relatively low cost, and ready availability.19 Therefore, large amount of investigates were performed on the synthesis of polyols from vegetable oils such as sunflower,20 rapeseed oil,21 and soybean oil,22 linseed oil,23 palm kernel oil,24 and castor oil.25 From vegetable oil polyols, several PU materials such as coatings, adhesives, sealants and elastomers are obtained. Nonetheless, for extraordinary application, these PU materials need to be endowed with excellent and special performance. As a result, a growing number of work have been conducted, for instance, silicon-containing compounds including polysiloxanes and POSS were utilized as modifier. Raftopoulos26 prepared PU/POSS hybrids with aminoethylaminopropyl isobutyl POSS (DIAPOSS) and 1,2-propanediol isobutyl POSS (PHIPOSS), and Tg of the materials was greatly improved. Mather and co-workers27 obtained poly(ε-caprolactone)–POSS by controlling the molar ratio of PCL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POSS. In addition, other POSS equipped with active groups such as hydroxyl-group and amino-group were also successfully introduced to polyurethane chains to pursuit of higher quality of material.28
POSS. In addition, other POSS equipped with active groups such as hydroxyl-group and amino-group were also successfully introduced to polyurethane chains to pursuit of higher quality of material.28
From these points of view, to solve the global emergency environmental problems and overcome the shortcoming of pure vegetable oil-based polyurethanes, we propose a synthetic method of novel green polyol to replace petrol-based polyol, at the same time, DDSQ was introduced in the main chain. Meanwhile, we also discussed the mechanical properties, thermal properties, the surface properties, the crystallinity and morphology of these hybrid polyurethanes after modification. The results of this paper can provide some insight and scientific data for vegetable oil-based hybrid polyurethanes.
2. Experimental section
2.1. Materials
Epoxy soybean oil (ESO) with the epoxy value 6.1% was obtained from Hairma Chemical (GZ) Ltd., China. Phenyltrimethoxysilane (98%) was purchased from Xiya Reagent Co. (Chengdu China) and used as received, isophorone diisocyanate (IPDI) was purchased from Wuxi East Grace Electronic Material Technology Co., Ltd. Prior to use, reagents such as tetrahydrofuran (THF) purified by refluxing above metal sodium and then distilled and stored in the presence of a molecular sieve of 4 Å molecular sieves to remove water. Ethyl acetate (EtAc), was dehydrated and also stored in the presence of 4 Å molecular sieves. Unless specially indicated, other reagents in this article were purchased from Shanghai Reagent Co., China and used without further purification.2.2. Synthesis
Then, the organic layer was purified with deionized water for three times, drying over MgSO4 and filtering, resulting in clear, light yellow castor oil fatty acid (COFA). Finally, the soy-castor oil based polyols (SCP) was prepared with a solvent-free and catalyst-free method through ring opening reaction between ESO and COFA.29 Specifically, COFA and ESO were mixed in a flask at the mole ratio of carboxyl to epoxy group was 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a magnetic stirrer and maintained at 170 °C in dry nitrogen atmosphere. After several hours, a light reddish/yellow, viscous liquid with a OH number 160 mg KOH per g was obtained. The preparation of representative green polyols was showed in Scheme 2.
1 with a magnetic stirrer and maintained at 170 °C in dry nitrogen atmosphere. After several hours, a light reddish/yellow, viscous liquid with a OH number 160 mg KOH per g was obtained. The preparation of representative green polyols was showed in Scheme 2.
| Samples | SCP mass (g) | IPDI vol. (mL) | Stannous octoat vol. (μL) | DDT8OH mass (g) | DDT8OH (wt%) |
|---|---|---|---|---|---|
| Pure PU | 4.00 | 1.26 | 100 | 0 | 0 |
| PU-1 | 3.87 | 1.26 | 100 | 0.10 | 1.88 |
| PU-2 | 3.74 | 1.26 | 100 | 0.20 | 3.79 |
| PU-3 | 3.48 | 1.26 | 100 | 0.40 | 7.68 |
| PU-4 | 3.21 | 1.26 | 100 | 0.60 | 11.66 |
| PU-5 | 2.95 | 1.26 | 100 | 0.80 | 15.73 |
2.3. Measurements
A Bruker Instruments (model Avance 400, Germany) at 400 MHz was used to record the 1H NMR spectroscopic analysis of the polymers. The measurements of polyols and DDT8OH were made using CDCl3 and acetone-d6 as solvent, respectively. Matrix-assisted ultraviolet laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF-MS) experiment was carried out on an ultrafleXtreme MALDI TOF/TOF mass spectrometer. The matrix, 2,5-dihydroxybenzoic acid, dissolved in THF (50 mg mL−1) was mixed with double-decker POSS solution (0.1 mg mL−1 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ratio). Nicolet 6700 infrared spectrometer was used to obtain the ATR-FTIR spectra. All spectra were carried out between 4000 cm−1 and 500 cm−1 with averaging 32 scans at a resolution of 4 cm−1. Scanning electron microscopy (S-4800, Hitachi) was applied to observe the morphology of the samples, and all the samples need to fractured with liquid nitrogen and coated with gold. Dynamic mechanical analysis (DMA) and thermogravimetric analysis (TGA) of the hybrid polyurethanes were performed using TA Instruments (DMA Q800) and (TGA/1100SF), respectively. The thermogravimetric analysis (TGA) was ramped at 20 °C min−1 under a nitrogen flow rate of 50 mL min−1 from 25 °C to 600 °C. While for DMA analysis, samples were cut from the cast films to feature dimensions of 10 (length) × 2 (width) × 0.5 mm (thickness). The apparatus was employed in tensile mode with a preload force of 10 mN, amplitude of 15 μm, and samples were tested from −70 °C to 150 °C at a rate of 3 °C min−1. The tensile properties were measured according to ASTM D 882-97 on a tensile tester model and the gauge length was 4 mm. The extension rate was 10 mm min−1, and five specimens were used for each sample. The static contact angles were carried out on a DCA-315 static contact angles, and ultrapure water and glycol were used as probe liquids at room temperature. All samples were measured at three different positions and the results were expressed as mean value. X-ray diffraction (XRD) analyses of the samples were carried out on a X-ray diffractometer (D8 Advance, Bruker AXS) using Cu-Kα radiation. The 2θ angle ranged from 3° to 60° at the scanning rate of 4° min−1.
1 v/v ratio). Nicolet 6700 infrared spectrometer was used to obtain the ATR-FTIR spectra. All spectra were carried out between 4000 cm−1 and 500 cm−1 with averaging 32 scans at a resolution of 4 cm−1. Scanning electron microscopy (S-4800, Hitachi) was applied to observe the morphology of the samples, and all the samples need to fractured with liquid nitrogen and coated with gold. Dynamic mechanical analysis (DMA) and thermogravimetric analysis (TGA) of the hybrid polyurethanes were performed using TA Instruments (DMA Q800) and (TGA/1100SF), respectively. The thermogravimetric analysis (TGA) was ramped at 20 °C min−1 under a nitrogen flow rate of 50 mL min−1 from 25 °C to 600 °C. While for DMA analysis, samples were cut from the cast films to feature dimensions of 10 (length) × 2 (width) × 0.5 mm (thickness). The apparatus was employed in tensile mode with a preload force of 10 mN, amplitude of 15 μm, and samples were tested from −70 °C to 150 °C at a rate of 3 °C min−1. The tensile properties were measured according to ASTM D 882-97 on a tensile tester model and the gauge length was 4 mm. The extension rate was 10 mm min−1, and five specimens were used for each sample. The static contact angles were carried out on a DCA-315 static contact angles, and ultrapure water and glycol were used as probe liquids at room temperature. All samples were measured at three different positions and the results were expressed as mean value. X-ray diffraction (XRD) analyses of the samples were carried out on a X-ray diffractometer (D8 Advance, Bruker AXS) using Cu-Kα radiation. The 2θ angle ranged from 3° to 60° at the scanning rate of 4° min−1.
3. Results and discussion
3.1. Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Besides, the resonance at 2.90 ppm was ascribed to the protons of hydroxyl groups. To sum up, these values were successfully consistent with the value calculated according to the structure formula. MALDI-TOF-MS was utilized to measure the molecular weight of DDT8OH and the mass spectrum was presented in Fig. 2.
10. Besides, the resonance at 2.90 ppm was ascribed to the protons of hydroxyl groups. To sum up, these values were successfully consistent with the value calculated according to the structure formula. MALDI-TOF-MS was utilized to measure the molecular weight of DDT8OH and the mass spectrum was presented in Fig. 2.
It is seen that the DDT8OH possessed the molecular weight of 1068 [1091.08–23], which was fully in accordance with the value calculated according to the structure formula. In short, the results of MALDI-TOF-MS and 1H NMR indicated the successful synthesis of DDT8OH.
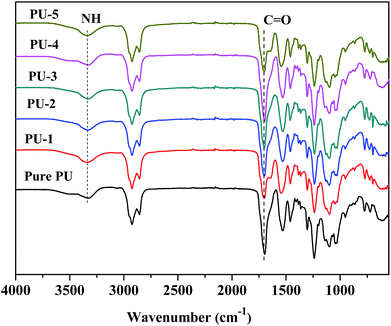
Fig. 4 showed the 1H NMR spectra of ESO and SCP, the peaks between 2.8 and 3.2 ppm were vanished, corresponding to the epoxy groups.30 Besides, new peaks were seen between 4.6 and 5.0 ppm, representing tertiary hydrogen atoms adjacent to the newly formed ester groups.31 Peaks corresponding to hydrogen bonded to carbons adjacent to the ester overlap with the peaks from the hydrogen attached to the carbon adjacent to OH (indicated as peak 3). The IR spectra of ESO and SCP were shown in Fig. 5. The epoxy group in the ESO was acknowledged by the peaks around 823 cm−1. However, after ring opening reaction with COFA, the peaks almost disappeared and new characteristic abroad absorption peaks around 3430 cm−1 appeared, which were attributed to the hydroxy groups. In conclusion, all these results indicated the successful conversion of ESO to soy-castor oil based polyols (SCP).
3.2. Thermal decomposition behavior
The thermal stability of the hybrid polyurethanes was evaluated by the thermogravimetric analysis (TGA) and the TG curves were shown in Fig. 8. All the hybrid polyurethanes displayed TGA profiles similar to the pure polyurethane, suggesting that the degradation mechanism did not significantly alter despite of the introduction of DDT8OH. For convenience, the onset of decomposition temperature was defined as Td5, where the samples attained 5% weight loss. The Td5 of hybrid polyurethanes took place above 200 °C, due to the decomposition of urethane bonds. Afterwards, soybean oil chain scission occurred at 340 °C (ref. 32) which acted as the second decomposition process. On the one hand, compared with the pure polyurethanes, the 5% weight loss temperatures (Td5) for hybrid polyurethanes were significantly higher, especially with 7.68% DDSQ content, whose Td5 even increased to 263 °C, almost 31 °C higher than pure polyurethanes. On the other hand, with the DDSQ content beyond 7.68%, the onset of decomposition temperature was inversely proportional to the concentration of DDSQ. Two reasons could explain this thermal phenomenon. Firstly, the hybrid polyurethanes with the inclusion of DDSQ displayed excellent thermal stability, due to the DDSQ accessed by means of chemical bonds in the main chain of the polyurethanes, which could significantly retard the movement and scission of molecular chains. Secondly, with the DDSQ content above 7.68 wt%, the aggregation of DDSQ monomer became seriously, which greatly reduced the effectiveness in preventing chains scission. In addition, the decomposition rates during the second decomposition process of the hybrid polyurethanes were slower, which could observed from the curves became flatter above 350 °C of high DDSQ content. At high temperature, the silicon dioxide from the oxidation of DDSQ would be wrapped in the surface so that the release of gaseous products from segmental decomposition was suppressed. Which significantly improved the thermal insulation and the flame resistance of the material.33 However, the increased residue yields of degradation were responsible for the formation of the silica from the thermal oxidation of silsesquioxanes in the process of degradation and decomposition (see Table 2).| Samples | DDT8OH (wt%) | Td5 (°C) | Char residue (wt%) | Tg (°C) |
|---|---|---|---|---|
| Pure PU | 0 | 232 | 2.46 | 26.8 |
| PU-1 | 1.88 | 241 | 2.49 | 41.2 |
| PU-2 | 3.79 | 256 | 4.93 | 53.3 |
| PU-3 | 7.68 | 263 | 7.62 | 78.2 |
| PU-4 | 11.66 | 260 | 11.55 | 76.4 |
| PU-5 | 15.73 | 252 | 10.82 | 74.3 |
3.3. DMA analysis
The hybrid polyurethanes were subjected to dynamic mechanical analysis (DMA) to measure the glass transition temperatures (Tg), and the glass transition temperature (Tg) could be evaluated by the peak of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ. All Tg values were listed in Table 2 and the DMA curves were shown in Fig. 9. PU-3, PU-4 and PU-5 showed only one peak which belonged to the soft segments, as for PU, PU1 and PU2, it was seen that a shoulder peak appeared on the left side of the main peak, but in general, they also owned the main peak belonged to the soft segments like the other PUs. With the DDSQ contents below 7.68 wt%, the value of Tg increased regularly as DDSQ content increased. Tg value of PU-3 (with 7.68% DDSQ) even reached 78.2 °C, almost 51.4 °C higher than that of pure PU. This observation suggested that the present of DDSQ, due to their nanophase dispersing and due to the chemical incorporation to the polyurethane chains, effectively hindered the motion of the network, thus the Tg of the network increased. In addition, as DDSQ loadings increased, high POSS content led to serious aggregation, which resulted in the increase of the free volume, because the aggregation particles acted as solid lubricant and caused the decrease of the Tg. As shown in the figure, the storage modulus of the PUs exhibited the three stages of change. At low temperature stage, the storage modulus basically remain unchanged with the temperature increased. However, with the temperature reached a certain degree, storage modulus sharply decreased to zero, the temperature zone is very narrow, and the value of glass transition temperature was on the range; continue to raise the temperature, the storage modulus was also maintain zero.
δ. All Tg values were listed in Table 2 and the DMA curves were shown in Fig. 9. PU-3, PU-4 and PU-5 showed only one peak which belonged to the soft segments, as for PU, PU1 and PU2, it was seen that a shoulder peak appeared on the left side of the main peak, but in general, they also owned the main peak belonged to the soft segments like the other PUs. With the DDSQ contents below 7.68 wt%, the value of Tg increased regularly as DDSQ content increased. Tg value of PU-3 (with 7.68% DDSQ) even reached 78.2 °C, almost 51.4 °C higher than that of pure PU. This observation suggested that the present of DDSQ, due to their nanophase dispersing and due to the chemical incorporation to the polyurethane chains, effectively hindered the motion of the network, thus the Tg of the network increased. In addition, as DDSQ loadings increased, high POSS content led to serious aggregation, which resulted in the increase of the free volume, because the aggregation particles acted as solid lubricant and caused the decrease of the Tg. As shown in the figure, the storage modulus of the PUs exhibited the three stages of change. At low temperature stage, the storage modulus basically remain unchanged with the temperature increased. However, with the temperature reached a certain degree, storage modulus sharply decreased to zero, the temperature zone is very narrow, and the value of glass transition temperature was on the range; continue to raise the temperature, the storage modulus was also maintain zero.
3.4. Morphology
Scanning electron microscopy (SEM) applied to investigate on the changes in surface morphology related to the DDSQ contents. Fig. 10 showed a group of SEM images of the samples with 3.79, 7.68, 11.66 and 15.73 wt% DDSQ, and it could be seen that the images of the composites displayed numerous micron-sized particles on the fracture surfaces. The number as well as the size of particles increased as DDSQ loading increased. At low DDSQ content, the morphology of the PU hybrid still displayed relatively smooth structure. However as DDSQ loading increased, the dispersed particles gradually seeped into the organic matrix and small particles gradually aggregated into larger ones. The phenomenon occurred because DDSQ had a strong aggregation effect through physical interactions. For these strong physical interactions, it might be interpreted that due to benzene ring being hydrophobic and inert to PU chains, the only direction of the nano-cages interacting with the matrix would be the hydroxyls hung on the open face of the DDSQ cage.3.5. Mechanical properties of hybrid polyurethanes
The stress–strain curves of hybrid polyurethanes were shown in Fig. 11. The pure polyurethanes exhibited brittle fracture which could owed to the changes of macromolecular bonds and angles. Low DDSQ content (not above 7.68 wt%) hybrid polyurethanes owned the large elongation at break but low tensile stress. However, when the content of DDSQ was above 11.66 wt%, the polymer chain segment started to rearrange and led to an unexpected yield point in PU-4 and PU-5. To sum up, the hybrid polyurethanes behaved like glassy polymer. Although the hybrid polyurethanes with low DDSQ content could both improve the tensile stress and maintain a certain elongation at break, but when the content of DDSQ exceeded 11.66 wt%, the tensile stress increased sharply and elongation at break declined sharply, either. Two reasons led to this phenomenon, firstly, with the chemical introduction of DDSQ, the cross-link density of the network increased. Besides, the DDSQ cages on the polymeric matrices existed the force of nano-reinforcement. The interaction of the two factors led to mechanical enhancement. Secondly, because of over-crosslinking and serious aggregation, the mechanical impairment occurred at higher DDSQ content.3.6. Surface hydrophobicity
It was necessary to investigate the surface wettability of the hybrid polyurethanes with DDSQ in the main chains, because DDSQ cages in the main chains of polyurethanes were the derivatives of the organosilicon compound, which was known as low free energy. So contact angles was applied to verify this effect. Probe liquids choose water and ethylene glycol, respectively. The results of static contact angles were summarized in Table 3. For pure polyurethane, the static contact angle of water was 99.3°, which already had a good hydrophobic due to the hydrophobicity of vegetable oil-based polyols itself.| Samples | DDT8OH (wt%) | Static contact angle | |
|---|---|---|---|
| θH2O (deg) | θethylene glycol (deg) | ||
| Pure PU | 0 | 99.3 ± 1.21 | 86.2 ± 0.40 |
| PU-1 | 1.88 | 101.2 ± 1.34 | 78.7 ± 0.10 |
| PU-2 | 3.79 | 106.4 ± 1.42 | 76.5 ± 0.30 |
| PU-3 | 7.68 | 115.2 ± 1.34 | 75.7 ± 0.20 |
| PU-4 | 11.66 | 121.2 ± 1.36 | 74.8 ± 0.20 |
| PU-5 | 15.73 | 128.5 ± 1.54 | 70.1 ± 0.10 |
However, with the inclusion of DDSQ, the contact angles of water was mildly heightened. Conversely, the contact angles of ethylene was decreased. Fig. 12 has clearly illustrated this. Even more unexpected, with the DDSQ content of 15.73 wt%, the water contact angle even reached to 128.5°, which strongly illustrated that the hydrophobicity of the hybrid polyurethanes were significantly improved.
3.7. Crystallinity
The crystallinity of PUs was investigated on pure PU, PU-2 and PU-5. The results were depicted in Fig. 13.As can be observed from the X-ray diffraction patterns, all the diffractograms showed well defined diffraction peak around 18.9° (main diffraction peak), which indicates the presence of the crystalline structure of PU. Besides, the X-ray patterns of PU-2 and PU-5 also exhibited the diffraction peak, which indicated the crystalline structure without damage with the introduction of DDSQ. Moreover, the intensity of the diffraction peak around 18.9° decreased with increasing DDSQ content, implying that the crystallinity of PU films gradually decreased. The Si–O–Si crosslinked network structure, which restricted the movement and ordered arrangement of chain segments, decreased the regularity of soft-segments, and therefore led to the decrease in the crystallinity of soft-segments.
4. Conclusions
In this work, a novel method was developed to prepare soy-castor oil based polyols for the application of polyurethane industry. The hybrid polyurethanes were synthesized with double-decker octaphenylsilsesquioxanetetraol (DDT8OH) as the raw materials partly replaced soy-castor oil based polyols (SCP) in one-step method. The molecular structures of the SCP and DDT8OH were characterized by 1H NMR, MALDI-TOF-MS and IR. According to the analysis, the soy-castor oil based polyols (SCP) and the DDT8OH were successfully synthetized. Meanwhile, the properties of the hybrid polyurethanes were evaluated by a series of characterizations. Thermogravimetric analysis (TGA) revealed that the stability of the hybrid polyurethanes were improved in terms of the high temperatures and the yields of degradation residues. The DMA results indicated that the hybrid polyurethanes exhibited increased glass transition temperatures (Tg) compared with the pure polyurethanes. SEM indicated that POSS was homogeneously dispersed in the polymer matrix at low DDT8OH concentration, however serious aggregation would occurred with high loading. As for the tensile tests, the hybrid polyurethanes showed enhanced tensile stress compared to the pure polyurethanes. In terms of surface properties, the hydrophobicity of the hybrid polyurethanes was significantly enhanced. Furthermore, the X-ray diffraction studies revealed that the cages of DDSQ maintain a self-assembling ability when incorporated to the hard segments of the polyurethane, and formed nanocrystalline phase. Overall, this new method provided a feasible way to prepare novel polyols for PUs with the inclusion of organic–inorganic double-decker silsesquioxane to own promising properties, and it is expected to partially or completely replace petroleum-based polyols in the PU industry. It will open up new pathways for the development of environmentally friendly polymer finally.Acknowledgements
This work was supported by research grants from National Key Technology Research and Development Program (2012BAD32B03-4), the Fundamental Research Funds for the Central Universities (JUSRP51623A) and the Cooperative Innovation Foundation of Industry, Academy and Research Institutes (BY2013015-10) in Jiangsu Province of China.References
- S. Wu, M. Kakimoto and H. Oikawa, Macromolecules, 2008, 41, 3481–3487 CrossRef CAS.
- S. Wei and F. C. Changb, Prog. Polym. Sci., 2011, 36, 1649–1696 CrossRef.
- R. Tamaki, J. Choi and R. M. Laine, J. Am. Chem. Soc., 2001, 123, 12416–12417 CrossRef CAS PubMed.
- M. Ak, B. Gacal, B. Kiskan, Y. Yagci and L. Toppare, Polymer, 2008, 49, 2202–2210 CrossRef CAS.
- H. Xu, S. Guang and C. Li, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 5308–5317 CrossRef CAS.
- D. Gnanasekaran, P. A. Walter and B. S. Reddy, Polym. Eng. Sci., 2013, 53, 1637–1644 CAS.
- K. Wu, B. K. Kandola, E. Kandare and Y. Hu, Polym. Compos., 2011, 32, 378–389 CrossRef CAS.
- J. C. Huang, C. B. He, K. Y. Mya, J. Dai and Y. P. Siow, Polymer, 2003, 44, 4491–4499 CrossRef CAS.
- H. Liu and S. Zheng, Macromol. Rapid Commun., 2005, 26, 196–200 CrossRef CAS.
- R. O. R. Costa, R. Tamaki and R. M. Laine, Macromolecules, 2001, 34, 5398–5407 CrossRef CAS.
- Y. Ni, S. Zheng and K. Nie, Polymer, 2004, 45, 5557–5568 CrossRef CAS.
- K. Zeng, Y. Liu and S. Zheng, Eur. Polym. J., 2008, 44, 3946–3956 CrossRef CAS.
- Y. Liu and S. Zheng, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1168–1181 CrossRef CAS.
- K. W. Huang and S. W. Kuo, Macromol. Chem. Phys., 2010, 211, 2301–2311 CrossRef CAS.
- S. Wu, M. A. Kakimoto and H. Oikawa, Macromolecules, 2007, 40, 5698–5705 CrossRef CAS.
- M. A. Hoque, S. Shinke and Y. Kawakami, Macromolecules, 2009, 42, 3309–3315 CrossRef CAS.
- J. Huang and P. Jiang, J. Mater. Sci., 2016, 51, 2443–2452 CrossRef CAS.
- S. Hu and Y. Li, Ind. Crops Prod., 2014, 57, 188–194 CrossRef CAS.
- C. K. Williams and M. A. Hillmyer, Polym. Rev., 2008, 48, 1–10 CrossRef CAS.
- Y. H. Hu, Y. Gao and D. N. Wang, J. Appl. Polym. Sci., 2002, 84, 591–597 CrossRef CAS.
- C. Yang, Z. H. Zhuang and Z. G. Yang, J. Appl. Sci., 2013, 131, 1–7 Search PubMed.
- Z. S. Petrovic, A. Zlatanic and W. Zhang, J. Appl. Polym. Sci., 2007, 105, 2717–2727 CrossRef CAS.
- S. Yadav, F. Zafar, A. Hasnat and S. M. Ahmad, Prog. Org. Coat., 2009, 64, 27–32 CrossRef CAS.
- K. H. Badri and S. H. Ahmad, J. Appl. Polym. Sci., 2001, 81, 384–389 CrossRef CAS.
- S. Thakur and N. Karak, Prog. Org. Coat., 2013, 76, 157–164 CrossRef CAS.
- K. N. Raftopoulos, P. Pissis and K. Pielichowski, Polymer, 2013, 54, 2745–2754 CrossRef CAS.
- E. H. Rattinger, K. Ishida, A. R. Uribe and P. T. Mather, Polymer, 2013, 54, 3350–3362 CrossRef.
- S. Turri and M. Levi, Macromolecules, 2005, 38, 5569–5574 CrossRef CAS.
- C. Zhang, Y. Xia, R. Chen and S. Huh, Green Chem., 2013, 15, 1477–1484 RSC.
- Y. S. Lu and R. C. Larock, Biomacromolecules, 2007, 8, 3108–3114 CrossRef CAS PubMed.
- Y. Xia and R. C. Larock, Polymer, 2010, 51, 2508–2514 CrossRef CAS.
- R. Gu, S. Konar and M. Sain, J. Am. Oil Chem. Soc., 2012, 89, 2103–2111 CrossRef CAS.
- C. Sanchez, B. Julián, P. Belleville and M. Popall, J. Mater. Chem., 2005, 15, 35 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |