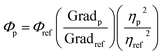Highly efficient ultraviolet light-emitting organosoluble polyimide†
Zhiyang Liu‡
ab,
Shouming Wu‡c,
Yongqi Baia,
Weigang Jiangab,
Ling Honga,
Tao Leiab and
Ziyi Ge*a
aNingbo Institute of Materials Technology & Engineering, Chinese Academy of Sciences (CAS), Ningbo, 315201, P. R. China. E-mail: geziyi@nimte.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing, 100049, P. R. China
cZhejiang Fluoride and Silicon Research Institutes, Quzhou, Zhejiang, P. R. China
First published on 19th July 2016
Abstract
We synthesized a new class of polyimides containing a siloxane unit in the main chain and showing an intense photoluminescence (PL) in the ultraviolet (UV) region at room temperature. The PL emission of the sample in solution was observed at around 313 nm with excitation at 266 nm. The fluorescence quantum yield was estimated to be 0.35. The polyimide in the film state also exhibited a strong PL emission around 328 nm. These results indicate that the synthesized polyimide has potential for UV light-emitting applications.
1. Introduction
Ultraviolet (UV) emission plays an important role in environmental protection, nano-fabrication technologies, and water and air purification/sterilization.1,2 In addition, UV gas sources have attracted considerable attention for potential uses in laser printing and high-density optical storage.3–5 Several UV light sources, including mercury lamps, excimer lasers, and second-harmonic generation lasers, are currently available. On the other hand, wide bandgap inorganic semiconductors, mainly group III nitrides such as AlN, GaN, and their ternary compounds, have been the main contenders of UV emitters for past several years.6–9 However, for realizing inexpensive and large area applications, polymers are more attractive than these two types of the light emitters, because of the lower efficiency of gas sources and the nonavailability of a large area single crystalline semiconductor.Polyimides, known for their excellent physical and chemical properties (for example, outstanding thermal resistances, good mechanical properties, high chemical stability, etc.), have been studied and developed for many years for both academic and technological interests.10–12 Various polyimides containing fluorescent dye groups in the main chain or side chains have been synthesized and characterized in order to fabricate luminescent polyimides. However, in spite of the fact that fluorescence dyes with a high fluorescence quantum yield (FQY, ∼1) are included, these polyimides showed only a small fraction of the FQY.13,14 Some semi-aromatic and wholly aromatic polyimides with aggregation induced emission effect possessed strong photoluminecent quantum yields.15–17
In this paper, we report on the synthesis and optical characterization of a polyimide containing siloxane. Notably, the non-conjugated polyimide without fluorescent dyes exhibited an intense photoluminescence (PL) peak around 313 nm with a high FQY of 0.35. A film of the polyimide also showed a similar PL emission peak around 328 nm, indicating its potential as a UV light source.
2. Results and discussion
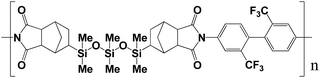
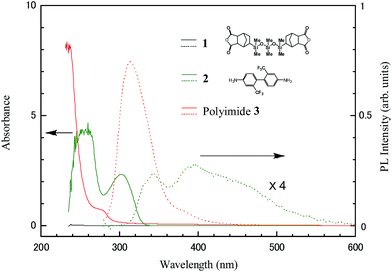
Organosoluble polysiloxaneimide (3) was synthesized from 5,5′-exo-(1,1,3,3,5,5-hexamethyl-trisiloxane-1,5-diyl)bisbicyclo[2,2,1]heptene-2,3-endo-dicarboxylic anhydride (1) and 2,2′-bis(trifluoromethyl)benzidine (2) by a one-step high-temperature polycondensation method according to reported procedures.18–20 Fig. 1 shows the chemical structure of the synthesized polyimide. The effects of the siloxane units in main chain on the properties of the polyimide were evaluated by solubility, thermal stability, optical absorption and PL measurements. The polyimide was readily soluble in polar aprotic solvents such as N-methylpyrrolidone, N,N-dimethylacetamide (DMAc), and chloroform at room temperature. It exhibited a glass transition temperature over 150 °C in N2 and a 5% weight loss at temperatures higher than 460 °C under air. The polyimide can be cast into flexible and tough films from a DMAc solution. The film has tensile strength of 48 MPa, a tensile modulus of 0.60 Gpa, and a percent elongation at break from 6.1%.Fig. 2 shows the UV-vis absorption and PL spectra of the two monomers (1 and 2) and the synthesized polyimide 3 dissolved in chloroform. The PL measurements were performed using the third harmonic line (266 nm) of a femto-second Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S laser operated with a repetition of 420 kHz as the excitation source. The excitation power was 50 μW. The spectra are corrected for the wavelength-dependent sensitivity of the detection system. Monomer 1 did not show any absorbance or any PL emission in the observed spectral range, which originates from non-conjugated bonds of the siloxane and carboxylic anhydride units. Monomer 2 exhibited absorption bands centered at ∼260 and ∼300 nm. These bands are attributed to the vibronic S0 → S1 transitions of the trifluoromethyl benzene units. The PL spectrum of the monomer 2 solution showed a S1 → S0 0–0 transition at ∼343 nm with associated higher vibronic transitions at longer wavelengths.
S laser operated with a repetition of 420 kHz as the excitation source. The excitation power was 50 μW. The spectra are corrected for the wavelength-dependent sensitivity of the detection system. Monomer 1 did not show any absorbance or any PL emission in the observed spectral range, which originates from non-conjugated bonds of the siloxane and carboxylic anhydride units. Monomer 2 exhibited absorption bands centered at ∼260 and ∼300 nm. These bands are attributed to the vibronic S0 → S1 transitions of the trifluoromethyl benzene units. The PL spectrum of the monomer 2 solution showed a S1 → S0 0–0 transition at ∼343 nm with associated higher vibronic transitions at longer wavelengths.
The absorption bands of polymer 3 shift to shorter wavelengths by about 30 nm compared to those of monomer 2. This blue-shift can be explained by the fact that the –NH2 bonds of the trifluoromethyl benzene unit are replaced by imide units in the polymer. With the blue-shift of absorption bands, the PL peak of the S1 → S0 0–0 transition also shifted to ∼313 nm, and its intensity became one order of magnitude greater than that of the PL emission from monomer 2. The full width at half maximum (FWHM) of the PL peak at ∼313 nm was estimated to be about 43 nm. This FWHM is narrower than that of other light-emitting polymers13,14 and monomer 2, which is in favour of the color purity. Compared to monomer 2, the narrow FWHM of polyimide 3 is due to the “locally excited” transition that occurs in the dianhydride moiety and the electron-donating –NH2 that was changed into the electron-accepting imide units resulting in the weak intramolecular “charge transfer” transition.21 The FQY of this UV emission was evaluated using tryptophan that possesses 0.14 of the FQY in water (pH 7.2 at 25 °C) as a reference material.22,23 As a result, an FQY of 0.35 was obtained (Fig. 3). To the best of our knowledge, this is the high FQY of UV emission not only from fluorescent polyimides, but also from light-emitting polymers.
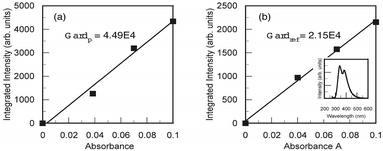 | ||
| Fig. 3 Integrated PL intensity plotted as a function of the absorbance for polyimide in chloroform (a) and L-tryptophan in water (b). | ||
Quenching of the PL intensity was observed for the monomer 2 solution at higher concentrations. This concentration quenching is understood to result from the intermolecular charge transfer effect.24,25 In contrast, the polyimide 3 solution emission intensity remained very stable. This result indicates that the siloxane and imide units act as a non-conjugated spacer, providing excellent resistance to UV radiation of the polyimide. The non-conjugated spacer may also suppress the contribution of the higher vibronic transitions and enhance the emission due to the S1 → S0 0–0 transition.
Finally, we investigated the optical properties of the film state of polyimide 3. Fig. 4 shows the absorption and PL spectra of a polyimide 3 film formed on a quartz substrate. The PL spectrum was obtained with an excitation power of 10 μW. The absorption spectrum of the film was slightly red-shifted from that of the solution and exhibited broad vibronic structures derived from the trifluoromethyl benzene units. The UV emission was also red-shifted relative to that of the solution, and it was observed at ∼328 nm, with a slightly broad FWHM (49 nm). We saw an apparent increase of the long wavelength tail arising from the higher vibronic transitions compared to that of the solution. This suggests a narrower distribution of emitting diamine units with increased effective conjugation lengths in the film state. The FQY of the polyimide 3 film is 0.11 with barium sulfate as a reference material, which is less than that of the polyimide 3 solution that is possibly owing to the molecular packing resulting in intermolecular charge transfer enhancement.21 The intense UV emission from the film state indicates that the synthesized polyimide has potential for UV light-emitting applications.
3. Experimental
Materials
5,5′-exo-(1,1,3,3,5,5-Hexamethyl-trisiloxane-1,5-diyl)bisbicyclo[2,2,1]heptene-2,3-endo-dicarboxylic anhydride (1) was synthesized according to a reported procedure.15–17 N,N-Dimethylacetamide (DMAc) (TCI) was purified by vacuum distillation over CaH2 prior to use. 2,2′-Bis(trifluoromethyl)benzidine (TFMB, 2) (TCI) was recrystallized from methanol and then dried at 50 °C for 2 h prior to use.Characterization
1H NMR, 13C NMR and 29Si NMR measurements were carried out by a JEOL JNM-AL 300 MHz spectrometer in CDCl3, acetone-d6, or DMSO-d6 without TMS. Infrared spectra were obtained using a JASCO Corp. FT-IR 460 Plus Fourier-transform infrared spectrophotometer. The ultraviolet-visible (UV-vis) spectra were recorded on a Shimadzu Corp. UV-3600 spectrophotometer at room temperature. The thickness of the specimen for Fourier transform infrared (FT-IR) and UV-vis measurements were controlled to be ∼10 μm. The inherent viscosities were determined at 0.5 g dL−1 concentration in a Kinematic TV-5S viscometer at 30 °C. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were carried out using a Seiko TGA 6200 and a Seiko DSC 6200 with a heating rate of 10 °C min−1 under air and nitrogen, respectively. Mechanical properties were measured on a TOYO BALDWIN CO. Ltd TENSILON/UTM-11-20 with a load cell of 5 kg and a 4 mm min−1 drawing speed. Tensile properties were calculated from stress–strain curves. The measurements were performed under ambient conditions with film specimens (0.5 cm wide, 6 cm long, thickness 20–30 μm), and the calculations were made with an average of at least five samples.Photoluminescence (PL) spectra of the solution and film were measured at room temperature using the third harmonic generation (266 nm) of a femto-second Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S laser pumped by a semiconductor laser diode. The pulse width was about 150 fs and the repetition frequency was 420 kHz. The excitation power was set at 50 and 10 μW for the solution and film, respectively. The PL emitted from the sample was collected by two achromatic lenses and then analyzed with a spectrograph equipped with a liquid-nitrogen-cooled CCD detector. All the spectra were corrected for the wavelength-dependent sensitivity of the used detection system.
S laser pumped by a semiconductor laser diode. The pulse width was about 150 fs and the repetition frequency was 420 kHz. The excitation power was set at 50 and 10 μW for the solution and film, respectively. The PL emitted from the sample was collected by two achromatic lenses and then analyzed with a spectrograph equipped with a liquid-nitrogen-cooled CCD detector. All the spectra were corrected for the wavelength-dependent sensitivity of the used detection system.
The fluorescence quantum yield (FQY) of the solution sample was evaluated using L-tryptophan which has 0.14 of the FQY in water (pH 7.2 at 25 °C), as the reference material. The PL spectra of polyimides were measured in chloroform and those for L-tryptophan were measured in water, with different absorbance (0.04, 0.07, and 0.1) at 266 nm respectively. From the measured spectra, the integrated intensity was calculated for each absorbance. Fig. 3(a) and (b) show the integrated PL intensity plotted as a function of the absorbance for the polyimide and L-tryptophan, respectively. The inset of Fig. 3(b) shows the PL spectrum of L-tryptophan. The gradient was evaluated based on Fig. 3. Finally, the fluorescence quantum yield of the polyimide solution was calculated using the following equation:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluent, yielding a yellow solid. The inherent viscosity of the resulting polyimide 3 was 0.38 dL g−1, measured at a concentration of 0.5 g dL−1 in DMAc at 30 °C. The polyimide film was prepared by casting the polyimide solution onto a quarts substrate, and then heating (100 °C/1 h, 180 °C/1 h, 250 °C/1 h) in vacuo. 1H-NMR (300 MHz, CDCl3): 7.69 (2H, br), 7.51 (2H, d, J = 9.3 Hz), 7.37 (2H, d, J = 9.3 Hz), 3.29 (4H, br), 2.89 (2H, br), 2.84 (2H, br), 1.73–1.62 (6H, m), 0.65 (2H, br), 0.08 (6H, s), 0.04 (6H, s), 0.01 (6H, s) ppm. 13C-NMR (75 MHz, CDCl3): 177.8, 177.7, 137.2, 133.3, 133.1, 130.7, 130.4 (F–CF2, J = 30.9 Hz), 129.4, 125.8, 124.9, 122.2 (ArH–CF3, J = 271.9 Hz), 52.2, 49.6, 42.3, 41.8, 40.9, 27.4, 26.6, 2.1, 0.0 ppm. 29Si-NMR (60 MHz, CDCl3): 5.95, −19.7 ppm. IR (KBr): ν = 1783, 1718 (imide C
3) as the eluent, yielding a yellow solid. The inherent viscosity of the resulting polyimide 3 was 0.38 dL g−1, measured at a concentration of 0.5 g dL−1 in DMAc at 30 °C. The polyimide film was prepared by casting the polyimide solution onto a quarts substrate, and then heating (100 °C/1 h, 180 °C/1 h, 250 °C/1 h) in vacuo. 1H-NMR (300 MHz, CDCl3): 7.69 (2H, br), 7.51 (2H, d, J = 9.3 Hz), 7.37 (2H, d, J = 9.3 Hz), 3.29 (4H, br), 2.89 (2H, br), 2.84 (2H, br), 1.73–1.62 (6H, m), 0.65 (2H, br), 0.08 (6H, s), 0.04 (6H, s), 0.01 (6H, s) ppm. 13C-NMR (75 MHz, CDCl3): 177.8, 177.7, 137.2, 133.3, 133.1, 130.7, 130.4 (F–CF2, J = 30.9 Hz), 129.4, 125.8, 124.9, 122.2 (ArH–CF3, J = 271.9 Hz), 52.2, 49.6, 42.3, 41.8, 40.9, 27.4, 26.6, 2.1, 0.0 ppm. 29Si-NMR (60 MHz, CDCl3): 5.95, −19.7 ppm. IR (KBr): ν = 1783, 1718 (imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1371 (C–N), 1261 (Si–Me), 1040 (Si–O–Si) cm−1.
O), 1371 (C–N), 1261 (Si–Me), 1040 (Si–O–Si) cm−1.4. Conclusion
In summary, a non-conjugated siloxane-containing polyimide, which exhibits an intense PL emission at ∼313 nm in chroloform excitated at 266 nm, was synthesized via a one-step high-temperature polycondensation method. The FQY of the solution state was estimated to be 0.35, which is the high value for a UV light-emitting polymer. The polyimide film still exhibited an intense UV emission. This is the first report of the synthesis of a non-conjugated siloxane-containing polyimide with an intense UV emission. Moreover, by introducing the siloxane units into the main chain, the film gains several characteristics, such as good mechanical properties, outstanding thermal resistance, and high organosolubility. Thus, the synthesized polyimide can be an attractive candidate for high performance UV solid-state light sources.Acknowledgements
This work was financially supported from the National Natural Science Foundation of China (21574144, 51273209, 51411140244), Zhejiang Provincial Natural Science Foundation of China (LR16B040002), CAS Interdisciplinary Innovation Team, and Ningbo Municipal Science and Technology Innovative Research Team (2015B11002, 2016B10005).References
- Y. Taniyasu, M. Kasu and T. Makimoto, Nature, 2006, 441, 325 CrossRef CAS PubMed
.
- A. Khan, Nature, 2006, 441, 299 CrossRef CAS PubMed
.
- H. Cao, X. Qiu, B. Luo, Y. Zhang, R. Tan, M. Zhao and Q. Zhu, Adv. Funct. Mater., 2004, 14, 243 CrossRef CAS
.
- M. H. Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo and P. Yang, Science, 2001, 292, 1897 CrossRef CAS PubMed
.
- Y. C. Kong, D. P. Yu, B. Zhang, W. Fang and S. Q. Feng, Appl. Phys. Lett., 2001, 78, 407 CrossRef CAS
.
- A. Khan, K. Balakrishnan and T. Katona, Nat. Photonics, 2007, 2, 77 CrossRef
.
- Y. Taniyasu and M. Kasu, Diamond Relat. Mater., 2008, 17, 1273 CrossRef CAS
.
- W. J. Sun, Y. L. Li, Y. Yang, Y. M. Li, C. Z. Gu and J. J. Li, J. Mater. Chem. C, 2014, 2, 2417 RSC
.
- E. C. Young, B. P. Yonkee, F. Wu, B. K. Saifaddin, D. A. Cohen, S. P. DenBaars, S. Nakamura and J. S. Speck, J. Cryst. Growth, 2015, 425, 389 CrossRef CAS
.
- G. Maier, Prog. Polym. Sci., 2001, 26, 3 CrossRef CAS
.
- J. Liu, Y. Nakamura, Y. Shibasaki, S. Ando and M. Ueda, Macromolecules, 2007, 40, 4614 CrossRef CAS
.
- A. Ghosh, S. K. Sen, S. Banerjee and B. Voitb, RSC Adv., 2012, 2, 5900 RSC
.
- S. M. Pyo, S. I. Kim, T. J. Shin, H. K. Park, M. Ree, K. H. Park and J. S. Kang, Macromolecules, 1998, 31, 4777 CrossRef CAS PubMed
.
- S. M. Pyo, S. I. Kim, T. J. Shin, M. Ree, K. H. Park and J. S. Kang, Polymer, 1998, 40, 125 CrossRef
.
- H.-J. Yen, C.-J. Chen and G.-S. Liou, Chem. Commun., 2013, 49, 630 RSC
.
- H.-J. Yen, J.-H. Wu, W.-C. Wang and G.-S. Liou, Adv. Opt. Mater., 2013, 1, 668 CrossRef
.
- J.-H. Wu, W.-C. Chen and G.-S. Liou, Polym. Chem., 2016, 7, 1569 RSC
.
- S. Wu, T. Hayakawa, R. Kikuchi, J. Stephen, M. Kakimoto and H. Oikawa, Macromolecules, 2007, 40, 5698 CrossRef CAS
.
- S. Wu, T. Hayakawa and M. Kakimoto, High Perform. Polym., 2008, 20, 281 CAS
.
- S. Wu, T. Hayakawa, M. Kakimoto and H. Oikawa, Macromolecules, 2008, 41, 3481 CrossRef CAS
.
- J. Wakita, H. Sekino, K. Sakai, Y. Urano and S. Ando, J. Phys. Chem. B, 2009, 113, 15212 CrossRef CAS PubMed
.
- E. P. Kirby and R. F. Steiner, J. Phys. Chem., 1970, 74, 4480 CrossRef CAS
.
- S. Dhami, A. J. de Mello, G. Rumbles, S. M. Bishop, D. Phillips and A. Beeby, Photochem. Photobiol., 1995, 61, 341 CrossRef CAS
.
- S. Blumstengel, I. Sokolik, R. Dorsinville, D. Voloschenko, M. He, O. Lavrentovich and L. C. Chien, Synth. Met., 1999, 99, 85 CrossRef CAS
.
- T. H. Tong and L. C. Chien, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 1450 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12755j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |