 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly improved dielectric properties of polymer/α-Fe2O3 composites at elevated temperatures
Kenichi
Hayashida
Materials and Processing Dept. II, Toyota Central R&D Labs., Inc., Nagakute, Aichi 480-1192, Japan. E-mail: e1440@mosk.tytlabs.co.jp
First published on 4th July 2016
Abstract
α-Fe2O3 particles were incorporated into ten kinds of polymer matrices, and the dielectric properties of the resultant polymer/α-Fe2O3 composites were investigated between 40 °C and 160 °C. We found that the dielectric properties strongly depended not only on the temperatures but also on the kind of polymer matrices. For engineering plastics such as polyetherimide (PEI), the dielectric constant ε′r was highly enhanced at around 1 kHz by incorporation of the α-Fe2O3 particles owing to Maxwell–Wagner polarization of free electrons in the α-Fe2O3 particles. This is probably because the π electrons in the aromatic structures of the engineering plastics strongly interact with the electrons in the α-Fe2O3 particles. Furthermore, the dielectric loss factor ε′′r for the engineering plastics became small at elevated temperatures because the conductivity of the α-Fe2O3 particle was enhanced and therefore the relaxation frequency of Maxwell–Wagner polarization was shifted to higher frequency. PEI/α-Fe2O3 composites exhibited highly improved dielectric properties at around 1 kHz, the high ε′r and very low ε′′r at the elevated temperatures above 120 °C. It was demonstrated that the PEI/α-Fe2O3 composite was comparable to the PEI/BaTiO3 composite in dielectric performance at 160 °C. Because the cost of α-Fe2O3 is much lower than that of BaTiO3, the PEI/α-Fe2O3 composite might be promising as a low-cost dielectric material for high-temperature applications.
Introduction
Dielectric materials are mainly used in capacitors to store electrical energy.1–3 In comparison to ceramic materials, polymer ones have the advantages of low cost, lightweight and high processability. However, the very low dielectric constant ε′r of the polymer material strongly prevents improvement of the capacitors. Therefore, polymer/ceramic composite materials have been much studied over the last few decades. Among the ceramics, BaTiO3 has been most widely used for enhancement in the ε′r of polymer materials because of the very high ε′r of BaTiO3.4–21 However, the high cost of BaTiO3 is a serious problem for industrial applications.On the other hand, hematite (α-Fe2O3) is a very low cost ceramic material although α-Fe2O3 has not been paid attention as a high ε′r filler for polymer materials owing to the low ε′r of α-Fe2O3; according to the reported literature, the ε′r of α-Fe2O3 is as low as 30.22–27 Therefore, few studies are available on the dielectric properties of polymer/α-Fe2O3 composites.28–30 Djoković and coworkers reported dielectric properties of a composites epoxy resin and α-Fe2O3 nanorods.28 Also, α-Fe2O3 nanorods were dispersed in a poly(vinyl alcohol)/poly(ethylene glycol) blend by Sayed et al.29 There are only small improvements of the ε′rs of the polymer materials in these studies.
In this paper, we focus on the conductivity σ of α-Fe2O3 at elevated temperatures. Typically, α-Fe2O3 is known to have σ of about 10−5 to 10−6 S cm−1 at room temperature.26 The σ of semiconductors such as α-Fe2O3 is enhanced as temperature is raised because electrons are excited from a valence band to a conductive band.31 It is expected that the σ of α-Fe2O3 is especially increased owing to its narrow band gap of 2.1 eV.32 At elevated temperatures, the enhanced σ of the α-Fe2O3 particles would bring a high ε′r to a polymer/α-Fe2O3 composite owing to Maxwell–Wagner polarization33 of the resultant free electrons in the α-Fe2O3 particles. The polymer/α-Fe2O3 composite might be promising as a low-cost dielectric material for high-temperature applications such as hybrid and electric vehicles.34,35 In this study, α-Fe2O3 particles were incorporated into ten kinds of polymer matrices, and the dielectric properties of the polymer/α-Fe2O3 composites were investigated between 40 °C and 160 °C. We found that the dielectric properties strongly depended not only on the temperatures but also on the kind of polymer matrices. Furthermore, the dielectric performance of one of the polymer/α-Fe2O3 composite was compared with that of a corresponding polymer/BaTiO3 composite at the elevated temperatures.
Experimental
Sample preparation
α-Fe2O3 particles were obtained from Kanto Chemical (Japan). The density and specific area of the α-Fe2O3 particles were determined to be 4.94 g cm−3 and 8.71 m2 g−1 using helium pycnometry and a standard BET method, respectively. The average diameter of the α-Fe2O3 particles was calculated to be 139 nm from the obtained density and specific area data. In the same manner, the density, specific area and average diameter of BaTiO3 particles obtained from Wako Pure Chemical Industries (Japan) were determined to be 5.73 g cm−3, 9.99 m2 g−1 and 105 nm, respectively.Polystyrene (PS) and poly(methyl methacrylate) (PMMA) were purchased from Wako Pure Chemical Industries (Japan). Poly(2-vinylpyridine) (P2VP) was obtained from Scientific Polymer Products. Styrene/acrylonitrile copolymer (SAN) with 30 wt% of acrylonitrile, polyphenylene ether (PPE), polysulfone (PSF), polyethersulfone (PES) and polyetherimide (PEI) were purchased from Sigma-Aldrich. Styrene/N-phenylmaleimide alternating copolymer (St-PMI)36 and polyarylate (PAR)37 were synthesized using reported methods, where PAR contained equimolar amounts of isophthaloyl and terephthaloyl moieties.
A series of polymer/α-Fe2O3 composites were prepared by simply blending the α-Fe2O3 particles with the polymer matrices. First, the α-Fe2O3 particles and the polymer matrix except PES were homogeneously dispersed in 1,4-dioxane by sonication. The dispersion was quickly frozen by liquid nitrogen, and then freeze-dried under vacuum. For preparation of PES/α-Fe2O3 composites, dichloromethane was used instead of 1,4-dioxane because PES was insoluble in 1,4-dioxane. Subsequently, all the pre-mixtures thus-obtained were further kneaded in molten states. Also, PEI/BaTiO3 composites were prepared in the same manner. The resultant composites were hot-pressed into disklike specimens with a diameter of 33 mm and a thickness of ∼0.52 mm. For electrical measurements, two gold electrodes with a diameter of 27 mm were deposited on the top and bottom of the specimens.
Measurements
Complex permittivity was obtained in the frequency f range of 102 to 106 Hz at 2 V using an LCR meter (E4980A, Agilent) coupled with a rheometer (ARES G2, TA instruments). The temperature of the specimen was controlled in the oven of the rheometer. Direct current (DC) resistance of the specimen was measured at room temperature under a nitrogen atmosphere using a megohmmeter (SM-8220, Hioki), and the value was recorded after 10 minute from application of a voltage of 500 V. The cross-section of the specimen that had been flattened with argon ion beam38 was observed using an SEM (S-4300, Hitachi) operated at an accelerating voltage of 2 kV.Results and discussion
Dielectric properties of polymer/α-Fe2O3 composites at 40 °C
We prepared a series of polymer/α-Fe2O3 composites with the volume fraction Φ of α-Fe2O3, Φ = 0.2. The chemical structures of the polymer matrices are shown in Fig. 1. All the polymers are popular, and some of them are engineering plastics that can be used over 150 °C (Fig. 1f–j). The ε′r and dielectric loss factor ε′′r values of the polymer/α-Fe2O3 composites (1 kHz, 40 °C) were listed in Table 1. There is a weak tendency for the ε′r of the composites prepared using the engineering plastics to be high. This is an interesting phenomenon that has never been reported. However, it is difficult to correctly evaluate the ε′r enhancement effect by direct comparing of the ε′r values of the composites because each ε′r of the polymer matrix ε′r polymer is different to some extent as shown in Table 1. Therefore, we suggest that apparent ε′r of α-Fe2O3, ε′r Fe2O3 is calculated using Lichteneker's logarithmic mixing rule and then compared in order to separate a contribution of the ε′r polymer itself from the ε′r of the composite. It is known that the ε′r of a polymer/filler composite often obeys Lichteneker's logarithmic mixing rule as follows:4,7,9,15–17,39| ε′r = ε′r polymer(ε′r filler/ε′r polymer)Φ, | (1) |
 | ||
| Fig. 1 Chemical structures of polymer matrices used in this study. (a) PS. (b) PMMA. (c) P2VP. (d) SAN. (e) St-PMI. (f) PPE. (g) PSF. (h) PAR. (i) PES. (j) PEI. | ||
| Polymer | ε′r | ε′′r | ε′r polymera | ε′r Fe2O3b |
|---|---|---|---|---|
| a ε′r of the polymer matrix only. b Apparent ε′r of α-Fe2O3 calculated using Lichteneker's logarithmic mixing rule. | ||||
| PS | 4.12 | 0.123 | 2.58 | 27.8 |
| PMMA | 5.25 | 0.453 | 3.39 | 31.2 |
| P2VP | 6.34 | 0.447 | 4.22 | 33.3 |
| SAN | 5.05 | 0.286 | 3.03 | 40.5 |
| PSF | 5.71 | 0.339 | 3.13 | 66.2 |
| St-PMI | 5.59 | 0.182 | 2.88 | 83.4 |
| PAR | 6.52 | 0.402 | 3.27 | 109 |
| PEI | 6.23 | 0.250 | 3.09 | 109 |
| PES | 7.13 | 0.475 | 3.52 | 116 |
| PPE | 5.58 | 0.162 | 2.63 | 120 |
The calculated ε′r Fe2O3 is shown in Table 1. There is a strong dependency of the ε′r Fe2O3 on the polymer matrix; the ε′r Fe2O3 is about 30 for PS, PMMA and P2VP whereas over 100 for PAR, PEI, PES and PPE. In other word, the ε′r for engineering plastics is more enhanced by the α-Fe2O3 particles than that for general-purpose plastics such as PS and PMMA. Fig. 2 shows the frequency f dependences of the ε′r and ε′′r of four kinds of composites for PEI, PSF, PPE and PS with Φ = 0.2 obtained at 40 °C. Every ε′r is decreased as f is increased. This is because the dielectric relaxation of a polarization occurs in the f range, which results in a ε′′r peak as shown in Fig. 2b. This polarization would be attributed to the interfacial polarization of free electrons in the α-Fe2O3 particles, that is Maxwell–Wagner polarization.33 Because the relaxation frequency becomes high as the σ of the α-Fe2O3 particles is high for Maxwell–Wagner polarization, it is suggested that the σ of the α-Fe2O3 particles in the PEI/α-Fe2O3 and PPE/α-Fe2O3 composites is higher than that in the PS/α-Fe2O3 composite, resulting in the higher ε′r Fe2O3 for PEI and PPE over the reported value of ∼30.22–27 This is probably because the π electrons in the aromatic structures of the engineering plastics strongly interact with the electrons in the α-Fe2O3 particles. Also, it is suggested that the enhanced ε′r Fe2O3 is caused by the dispersivity of the α-Fe2O3 particles in the polymer matrix and the interfacial state between the α-Fe2O3 particles and the polymer matrix. However, it is difficult to explain the strong frequency dependence of the dielectric properties as shown in Fig. 2 by these two contributions (the dispersivity and the interfacial state). Therefore, we conclude that the enhanced ε′r Fe2O3 is mainly due to the interaction between the electrons in the α-Fe2O3 particles and the polymer matrix. From the viewpoint of the molecular structures of the polymer matrix, the α-Fe2O3 particles might be more effective for fully aromatic polyester and polyimide with long conjugations.
Dielectric properties of polymer/α-Fe2O3 composites at 160 °C
The PEI/α-Fe2O3 and PPE/α-Fe2O3 composites are not useful at 40 °C owing to the large ε′′r values over 0.1 (Table 1). Under an electric field E, the power loss of a dielectric material per unit volume, P is expressed as,16| P = E2 × 2πfε0ε′′r, | (2) |
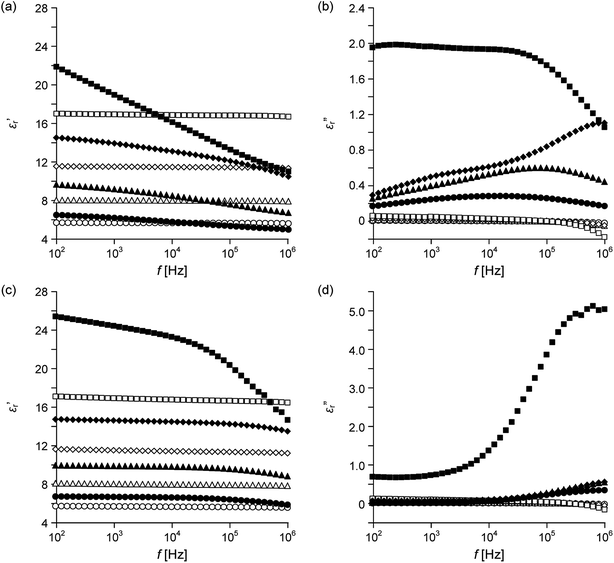
The large ε′′r values of the polymer/α-Fe2O3 composites are drastically reduced at 160 °C. Dielectric characteristics (1 kHz) obtained at 160 °C were listed in Table 2 for six kinds of polymer/α-Fe2O3 composites with Φ = 0.2. At 1 kHz, all the ε′′r values are less than 0.05. Furthermore, for PEI, PSF, PAR and PES, the ε′r Fe2O3 is more than 150 at 160 °C. For the four kinds of composites, the f dependences of the ε′r and ε′′r are shown in Fig. 3. Because the dielectric relaxations of Maxwell–Wagner polarization are not started at around 1 kHz as shown in Fig. 3b, the ε′′r values are small in the lower f ranges than 1 kHz. This result indicates the σ of the α-Fe2O3 particle is higher at 160 °C than 40 °C. Fig. 4 shows a variation in the f dependences of the ε′r and ε′′r of the PEI/α-Fe2O3 composites with Φ = 0.2 between 40 and 160 °C. The ε′′r peak is shifted to the higher f until the temperature reaches 120 °C.
| Polymer | ε′r | ε′′r | ε′r polymera | ε′r Fe2O3b |
|---|---|---|---|---|
| a ε′r of the polymer matrix only. b Apparent ε′r of α-Fe2O3 calculated using Lichteneker's logarithmic mixing rule. | ||||
| St-PMI | 5.95 | 0.0307 | 2.87 | 116 |
| PPE | 5.71 | 0.0110 | 2.61 | 139 |
| PEI | 6.73 | 0.0321 | 3.11 | 157 |
| PSF | 6.82 | 0.0525 | 3.12 | 165 |
| PAR | 7.29 | 0.0509 | 3.31 | 182 |
| PES | 8.32 | 0.0533 | 3.53 | 246 |
 | ||
| Fig. 4 Frequency f dependence of (a) ε′r and (b) ε′′r of a PEI/α-Fe2O3 composite with Φ = 0.2 obtained at 40 °C (circles), 80 °C (triangles), 120 °C (diamonds) and 160 °C (squares). | ||
For the four polymer matrices, we also prepared polymer/α-Fe2O3 composites with Φ = 0.4. Fig. 5 shows the f dependences of the ε′r and ε′′r of polymer/α-Fe2O3 composites with Φ = 0.4 at 160 °C. The ε′′r of the PEI/α-Fe2O3 composite remains small whereas for PAR and PES, the ε′′r becomes very large over all the f range. In order to elucidate the large ε′′r, the volume resistivity ρDC of the composites was measured. At Φ = 0.4, the ρDC for PAR and PES is much lower than that for PEI and PSF as shown in Fig. 6. ε′′r is the sum of a loss from σ, ε′′r(σ) and losses from dielectric polarizations ε′′r(DP):40
| ε′′r = ε′′r(σ) + ε′′r(DP). | (3) |
Furthermore, ε′′r(σ) is described using ρDC as follows:
| ε′′r(σ) = 1/(2πfε0ρDC). | (4) |
It is suggested that because ε′′r(σ) is inversely proportion to f and ρDC, the ε′′r is highly increased at the low f range for PAR and PES. The low ρDC for PAR and PES could be attributed to a leak current passing through the conductive networks of the α-Fe2O3 particles, that is, percolation of the α-Fe2O3 particles. According to the percolation theory with a random packing of spherical particles, the percolation threshold is calculated to be about 0.30.41,42 Therefore, it was reasonable that the percolation of the α-Fe2O3 particles occurred in the polymer/α-Fe2O3 composites with Φ = 0.4. To the contrary, it was unusual that the PEI/α-Fe2O3 composite had a high ρDC even at Φ = 0.4. We suspected that the dispersivity of the α-Fe2O3 particles in the polymer matrix was different between the PEI/α-Fe2O3 and PAR/α-Fe2O3 composites, and observed it using SEM. Fig. 7 shows SEM images of the PEI/α-Fe2O3 and PAR/α-Fe2O3 composites with Φ = 0.20 and Φ = 0.4. There is no great difference in the dispersivity of the α-Fe2O3 particles, which means that the significant difference in ρDC is not due to the dispersivity of the α-Fe2O3 particles. In the PEI/α-Fe2O3 composite, the α-Fe2O3 particles would be covered with a PEI layer at a nanometer scale, resulting in the high ρDC for PEI owing to inhibition of the tunneling conduction of the free electron39,43 between the α-Fe2O3 particles.
 | ||
| Fig. 7 SEM images of (a and b) PEI/α-Fe2O3 and (c and d) PAR/α-Fe2O3 composites with (a and c) Φ = 0.2 and (b and d) Φ = 0.4. | ||
Comparing of dielectric performance for PEI/α-Fe2O3 and PEI/BaTiO3 composites
As mentioned above, the PEI/α-Fe2O3 composite exhibits the high ε′r and very low ε′′r at the elevated temperature above 120 °C, which might be promising as a low-cost dielectric material for high-temperature applications; PEI has a maximum operating temperature of 200 °C.35 Therefore, the novel PEI/α-Fe2O3 composite was compared with a conventional PEI/BaTiO3 composite in dielectric performance. As shown in Fig. 6, the ρDC values of both the composites are comparable at room temperature when Φ is less than 0.4, whereas the ρDC of PEI/α-Fe2O3 is much lower than that of PEI/BaTiO3 at the higher Φ. This is because the interparticle distance becomes small enough for tunneling conduction to occur owing to high loading of particles. Fig. 8 shows the f dependences of the ε′r and ε′′r of the two types of composites obtained at 40 °C and 160 °C. At 40 °C, although the ε′r of PEI/α-Fe2O3 is higher than that of PEI/BaTiO3 in the lower f range, the ε′′r of PEI/α-Fe2O3 is much larger than that of PEI/BaTiO3. At 160 °C, however, ε′r has comparable ε′′r values to PEI/BaTiO3 except with Φ = 0.5, and higher ε′r than PEI/BaTiO3. The dielectric characteristics at 1 kHz obtained at 160 °C are plotted as a function of Φ in Fig. 9. These results show that the PEI/α-Fe2O3 composite was comparable to the PEI/BaTiO3 composite in dielectric performance at 160 °C. The fitting curves for the ε′r of the two types of composites are drawn in Fig. 9a in solid lines using Lichteneker's logarithmic mixing rule. These theoretical curves well fit to the experimental results and give ε′r BaTiO3 of 86 and ε′r Fe2O3 of 170, respectively. This value of 86 for BaTiO3 is consistent with some reported results; ε′r BaTiO3 is around 100.8,39Conclusion
The dielectric properties of polymer/α-Fe2O3 composites strongly depended not only on the temperatures but also on the kind of polymer matrices. For engineering plastics such as PEI, PSF, PAR and PES, the ε′r was highly enhanced at around 1 kHz by incorporation of α-Fe2O3 particles owing to Maxwell–Wagner polarization of free electrons in the α-Fe2O3 particles. This is probably because the π electrons in the aromatic structures of the engineering plastics strongly interact with the electrons in the α-Fe2O3 particles. Furthermore, the ε′′r for the engineering plastics became small at the elevated temperatures because the σ of the α-Fe2O3 particle was enhanced and therefore the relaxation frequency of Maxwell–Wagner polarization was shifted to higher f. PEI/α-Fe2O3 composites exhibited highly improved dielectric properties at around 1 kHz, the high ε′r and very low ε′′r at the elevated temperature above 120 °C. It was demonstrated that the PEI/α-Fe2O3 composite was comparable to the PEI/BaTiO3 composite in dielectric performance at 160 °C. Because the cost of α-Fe2O3 is much lower than that of BaTiO3, the PEI/α-Fe2O3 composites might be promising as a low-cost dielectric material for high-temperature applications.Acknowledgements
SEM observation was carried out by Mr Yasuhiro Takatani at Toyota Central R&D Labs Inc. The authors appreciate his help.References
- W. J. Sarjeant, J. Zirnheld, F. W. MacDougall, J. S. Bowers, N. Clark, I. W. Clelland, R. A. Price, M. Hudis, I. Kohlberg, G. McDuff, I. McNab, S. G. Parler Jr and J. Prymak, in Handbook of low and high dielectric constant materials and their applications, ed. H. S. Nalwa, Academic Press, London, 1999, vol. 2, ch. 9, pp. 423–491 Search PubMed.
- Q. Wang and L. Zhu, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 1421–1429 CrossRef CAS.
- Z. M. Dang, J. K. Yuan, J. W. Zha, T. Zhou, S. T. Li and G. H. Hu, Prog. Mater. Sci., 2012, 57, 660–723 CrossRef CAS.
- S. D. Cho, S. Y. Lee, J. G. Hyun and K. W. Paik, J. Mater. Sci.: Mater. Electron., 2005, 16, 77–84 CrossRef CAS.
- P. Kim, S. C. Jones, P. J. Hotchkiss, J. N. Haddock, B. Kippelen, S. R. Marder and J. W. Perry, Adv. Mater., 2007, 19, 1001–1005 CrossRef CAS.
- J. Lu and C. P. Wong, IEEE Trans. Dielectr. Electr. Insul., 2008, 15, 1322–1328 CrossRef CAS.
- K. W. Jang and K. W. Paik, J. Appl. Polym. Sci., 2008, 110, 798–807 CrossRef CAS.
- P. Kim, N. M. Doss, J. P. Tillotson, P. J. Hotchkiss, M. J. Pan, S. R. Marder, J. Li, J. P. Calame and J. W. Perry, ACS Nano, 2009, 3, 2581–2592 CrossRef CAS PubMed.
- Y. Kobayashi, A. Kurosawa, D. Nagao and M. Konno, Polym. Eng. Sci., 2009, 49, 1069–1075 CAS.
- A. Choudhury, Mater. Chem. Phys., 2010, 121, 280–285 CrossRef CAS.
- L. Xie, X. Huang, C. Wu and P. Jiang, J. Mater. Chem., 2011, 21, 5897–5906 RSC.
- S. Siddabattuni, T. P. Shuman and F. Dogan, Mater. Sci. Eng., B, 2011, 176, 1422–1429 CrossRef CAS.
- M.-F. Lin, V. K. Thakur, E. J. Tan and P. S. Lee, RSC Adv., 2011, 1, 576–578 RSC.
- Y. Song, Y. Shen, H. Liu, Y. Lin, M. Li and C. W. Nan, J. Mater. Chem., 2012, 22, 16491–16498 RSC.
- X. Wu, Z. Chen and Z. Cui, Compos. Sci. Technol., 2013, 81, 48–53 CrossRef CAS.
- K. Hayashida and Y. Matsuoka, Carbon, 2012, 60, 506–513 CrossRef.
- K. Hayashida, Y. Matsuoka and Y. Takatani, RSC Adv., 2014, 4, 33530–33536 RSC.
- C. Fettkenhauer, J. Glenneberg, W. Münchgesang, H. S. Leipner, G. Wagner, M. Diestelhorst, C. Pientschke, H. Beige and S. G. Ebbinghaus, RSC Adv., 2014, 4, 40321–40329 RSC.
- W. Lei, R. Wang, D. Yang, G. Hou, X. Zhou, H. Qiao, W. Wang, M. Tian and L. Zhang, RSC Adv., 2015, 5, 47429–47438 RSC.
- W. Yang, S. Yu, S. Luo, R. Sun, W.-H. Liao and C.-P. Wong, J. Alloys Compd., 2015, 620, 315–323 CrossRef CAS.
- Y. Feng, W. L. Li, Y. F. Hou, Y. Yu, W. P. Cao, T. D. Zhang and W. D. Fei, J. Mater. Chem. C, 2015, 3, 1250–1260 RSC.
- R. B. Hilborn Jr, J. Appl. Phys., 1965, 36, 1553–1557 CrossRef.
- K. Iwauchi, S. Yamamoto, Y. Bando and N. Koizumi, Bull. Inst. Chem. Res., Kyoto Univ., 1970, 48, 159–169 CAS.
- D. A. Sverjensky and N. Sahai, Geochim. Cosmochim. Acta, 1996, 60, 3773–3797 CrossRef CAS.
- M. L. Jimenez, F. J. Arroyo, F. Carrique and U. Kaatze, J. Phys. Chem. B, 2003, 107, 12192–12200 CrossRef CAS.
- J. A. Glasscock, P. R. F. Barnes, I. C. Plumb, A. Bendavid and P. J. Martin, Thin Solid Films, 2008, 516, 1716–1724 CrossRef CAS.
- R. A. Lunt, A. J. Jackson and A. Walsh, Chem. Phys. Lett., 2013, 586, 67–69 CrossRef CAS.
- D. Dudić, M. Marinović-Cincović, J. M. Nedeljković and V. Djoković, Polymer, 2008, 49, 4000–4008 CrossRef.
- A. M. E. Sayed and W. M. Morsi, J. Mater. Sci., 2014, 49, 5378–5387 CrossRef.
- D. Chen, H. Quan, Z. Huang, S. Luo, X. Luo, F. Deng, H. Jiang and G. Zeng, Compos. Sci. Technol., 2014, 102, 126–131 CrossRef CAS.
- C. Kittel, in Introduction to Solid State Physics, ed. C. Kittel, John Wiley & Sons, New York, 7th edn, 1996, ch. 8, pp. 197–232 Search PubMed.
- S. Mohanty and J. Ghose, J. Phys. Chem. Solids, 1992, 53, 81–91 CrossRef CAS.
- A. Schönhals and F. Kremer, in Broadband Dielectric Spectroscopy, ed. F. Kremer and A. Schönhals, Springer-Verlag, Berlin, 2003, ch. 3, pp. 59–98 Search PubMed.
- D. Tan, L. Zhang, Q. Chen and P. Irwin, J. Electron. Mater., 2014, 43, 4569–4575 CrossRef CAS.
- Q. Li, L. Chen, M. R. Gadinski, S. Zhang, G. Zhang, H. Li, A. Haque, L. Q. Chen, T. Jackson and Q. Wang, Nature, 2015, 523, 576–580 CrossRef CAS PubMed.
- M. N. Teerenstra, P. A. M. Steeman, W. Iwens, A. Vandervelden, D. R. Suwier, B. V. Mele and C. E. Koning, e-Polym., 2003, 3, 596–607 Search PubMed.
- H.-B. Tsai and Y.-D. Lee, J. Polym. Sci., Part A: Polym. Chem., 1987, 25, 1505–1515 CrossRef CAS.
- N. Erdman, R. Campbeli and S. Asahina, Microsc. Today, 2006, 14, 22–25 Search PubMed.
- K. Hayashida, RSC Adv., 2013, 3, 221–227 RSC.
- K. Hayashida and Y. Matsuoka, Carbon, 2015, 85, 363–371 CrossRef CAS.
- G. E. Pike and C. H. Seager, Phys. Rev. B: Solid State, 1974, 10, 1421–1434 CrossRef.
- C. D. Lorenz and R. M. Ziff, J. Chem. Phys., 2001, 114, 3659–3661 CrossRef CAS.
- K. Hayashida and H. Tanaka, Adv. Funct. Mater., 2012, 22, 2338–2344 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)