A marine algal polyphenol, dieckol, attenuates blood glucose levels by Akt pathway in alloxan induced hyperglycemia zebrafish model
Abstract
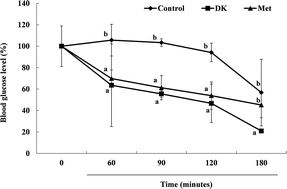
Recently, zebrafish (Danio rerio) have been used as a powerful model for human disease. The anti-diabetes activity of the anti-diabetic compound, dieckol (DK), one of the marine algal polyphenols isolated from Ecklonia cava, was confirmed in the zebrafish model. The zebrafish were divided to four groups, the normal (alloxan-untreated), alloxan-induced diabetic zebrafish without (control) and with DK, as well as those treated with metformin, a commercial drug. The blood glucose levels of the DK group of the hyperglycemic zebrafish at 90 min were decreased more than 3.3 times compared with the control group. Furthermore, reduced glucose-6-phosphate and phosphoenolpyruvate carboxykinase were observed with the treatments of DK and metformin in the liver tissues, and there was increased phosphorylation of protein kinase B (Akt) in the muscle tissue in the zebrafish model. Akt activation was involved in mediating the effect of DK on glucose transport activation and insulin sensitivity. These results prove that DK exerts a strong anti-diabetic effect by improving blood glucose regulation, hepatic glucose metabolic regulation and Akt up-regulation in alloxan induced hyperglycemic zebrafish.

 Please wait while we load your content...
Please wait while we load your content...