Precise casting of biomorphic La0.9K0.1CoO3 catalysts derived from pinewood for diesel soot combustion†
Abstract
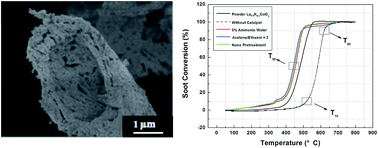
Porous biomorphic La0.9K0.1CoO3 catalysts were fabricated by the bio-templating method using tailored pinewood as the template and La0.9K0.1CoO3 sol as the precursor. The specimens were characterized by SEM, TEM, XRD, FT-IR, EDX, and N2-adsorption techniques. SEM and TEM results proved that the biomorphic cellular morphology of the initial pinewood structure was retained after heat treatment. The XRD and FT-IR results suggested a perovskite-type crystal structure for the microcellular catalysts. The generated catalysts possessed a relatively high surface area (147.97 m2 g−1) and porosity with a mean pore size of approximately 4–25 μm. The catalytic activity for the combustion of diesel soot was tested by a technique using a temperature-programmed reaction. The biomorphic La0.9K0.1CoO3 exhibited better catalytic activity for diesel soot combustion at low temperature than the La0.9K0.1CoO3 powders prepared from the same precursor sol due to enhanced contact efficiency between the soot and the catalysts, and the value of T10 was about 340 °C.

 Please wait while we load your content...
Please wait while we load your content...