DOI:
10.1039/C6RA12628F
(Paper)
RSC Adv., 2016,
6, 69454-69464
Separation of xylose using a thin-film composite nanofiltration membrane: screening of interfacial polymerization factors
Received
15th May 2016
, Accepted 15th July 2016
First published on 15th July 2016
Abstract
Most hydrolysis studies on biomass produce a high amount of xylose and glucose compared to other monosaccharides. A specially tailored thin-film composite (TFC) membrane prepared via interfacial polymerization (IP) using triethanolamine (TEOA) and trimesoyl chloride (TMC) as monomers on a polyethersulfone (PES) membrane was used to separate xylose from glucose. Differences between the support (PES) and TFC membrane in surface chemistry were probed using attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy and contact angle. Both membranes were also characterized by field emission scanning electron microscopy (FESEM) and pure water permeability to observe changes to membrane morphology and properties. The performance of the TFC membrane is highly stimulated by variation of preparative factors in IP. This study screens and reports the effect of five preparative factors, namely monomer concentrations (TEOA and TMC), pH of the aqueous phase, reaction time, and curing toward the performance of xylose separation from glucose. A 25−1 fractional factorial design was used to narrow down significant preparative factors, saving lots of time and resources. It was found that curing and reaction time significantly affected the separation of xylose from glucose. High correlation (R2 = 0.9998) between the experimental data and model data was obtained. The developed model in this study is adequate for predicting the xylose separation factor under different IP conditions within the range used. This study will provide valuable guidelines to develop membranes that are specially tailored for xylose separation from glucose as an alternative to the cost intensive chromatographic processes in use.
Introduction
Many monosaccharides are important ingredients in food and pharmaceutical industries. More often pure fractions of a specific monosaccharide are sometimes required.1 Many studies have been conducted in turning biomass especially generated from the oil palm sector, into monosaccharides by the various methods of pre-treatment and hydrolysis. The majority of hydrolysis studies on biomass available in Malaysia reported a high amount of xylose and glucose in hydrolysates compared to other monosaccharides.2–5
Most of the monosaccharides separation in industry is performed by chromatographic process. Chromatographic process requires a large amount of time, resources (usually water use for eluent), and energy.6,7 Significantly, commercial NF membranes (Desal-5 DK, Desal-5 DL and NF270) have demonstrated the ability to separate monosaccharides, especially xylose from glucose.8 NF processes offer a more cost-effective and easy-maintenance alternative separation of monosaccharides.8–10 In another study, an integrated membrane system using commercial membrane to perform enzymatic process, converting glucose to gluconic acid and followed by separation of xylose from gluconic acid by NF.11 However, development of TFC membrane tailored specifically for xylose separation from glucose has not been reported before.
Here, our focus is to specifically develop TFC membrane that has ability to separate xylose from glucose that can be directly applied on biomass hydrolysate. TFC membranes with ultrathin selective layer prepared using this IP technique is an excellent candidate for producing specially tailored TFC membrane for xylose and glucose separation. IP techniques gained popularity over the other coating techniques when a variety of TFC membrane was successfully developed by many companies, allowing wide application in the separation processes industry.12 TFC membrane developed using IP allows properties of both bottom substrate and top barrier film to be individually tailored and optimized to achieve desired separation selectivity and flux rate.13 The top thin layer produced by interfacial polymerization technique determines the overall solute retention, permeate flux, and, in general, controls the efficiency of the membrane process.14
Tang et al.15 had utilized TEOA, an economical monomer in enhancing the performance of the thin-film composite membrane. TEOA belong to the tertiary amino group, where its molecules can be flexibly transferred into quaternary ammonium group through variation of feed pH. Since, most hydrolysis of biomass occurs at low pH conditions. This unique property allows the membrane to increase hydrophilicity at lower pH feed, making it suitable for biomass hydrolysate application without additional steps. The increase in membrane hydrophilicity could enhance sugars separation since retention of a dissolved organic compound, such as sugar is influenced by both molecular size and hydrophobicity of the compound.16
Polysulfone appears to be the most popular polymer as support membrane for fabricating composite membranes.17,18 This is due to the advantages of polysulfone polymers which have properties such as high thermal resistance, wide pH tolerances, good chlorine resistance, flexibility in membrane fabrication, and high mechanical characteristics.19 Support membrane with high hydrophilicity weakens the adhesion between the active layer and the support membrane.18 Support layer with less hydrophilicity and larger pore produced more permeable composite membranes.17 Another polymer with similar properties as polysulfone but has slightly less hydrophilicity, like polyethersulfone may offer better adhesion and permeability.
The transport property (flux and rejection) of TFC membrane is mostly determined by the membrane intrinsic properties like surface charge, morphology, hydrophilicity/hydrophobicity, pore size and their geometry, and thickness of the thin-film. All these properties are influenced by the membrane preparation conditions like polymerization reaction time, curing temperature, curing time, monomer type and concentration.15,20–23 However, most of these studies employed one factor at a time (OFAT) design, where no statistical analysis was applied in investigating the effect of the factors.
A screening experiment usually involves only two levels of each factor and can also be called characterization testing or sensitivity analysis using multivariate tools such as two-level full factorial design.24 Fractional factorial designs permit investigation of the effects of many factors in fewer runs than a full factorial design.25 Till now, no report is available on factor screening approach for identifying significant factors particularly influencing xylose separation from glucose using TFC membrane.
The aims of the present work were to evaluate the effects of IP factors on xylose separation factor and to identify the key factors having significant impact on xylose separation. A 25−1 fractional factorial design were employed to define the most significant factors among concentration of monomers (TEOA and TMC), reaction time, pH of aqueous monomer, and curing which affect xylose separation performance. A model was then developed and analysed using analysis of variance (ANOVA) to identify the correlation between experimental data and predicted value. Numerical optimization was performed and validated to find the best conditions to obtain the maximum xylose separation factor using the developed model.
Materials and methods
Chemicals and reagents
The asymmetric commercial PES membrane was purchased from AMFOR Inc. (China) with the commercial name of UF PES50. The membrane has a nominal molecular cut-off of 50 kDa and water flux (at 25 °C) of 260 LMH. The chemicals used in this study were triethanolamine (R & M Marketing, Essex, UK), trimesoyl chloride (Alfa Aesar, UK), sodium hydroxide (Merck, Germany), n-hexane (Merck, Germany), xylose (Sigma Aldrich, USA), glucose (Sigma Aldrich, USA), and acetonitrile (J.T. Baker, USA). All chemicals were analytical grade with high purity (>99%) and acetonitrile with High Performance Liquid Chromatography (HPLC) grade.
Preparation of TFC membrane
The aqueous solution was prepared by dissolving sodium hydroxide in ultrapure water according to pH of aqueous solution as base medium for TEOA solution. TEOA is then dissolved in the sodium hydroxide solution. The organic solution was composed of TMC in the n-hexane. Firstly, commercial PES was soaked in an aqueous solution a period of 30 minutes. After that, the membrane was then drained and immersed in an organic solution for a certain period of time. Finally, the TFC membrane was dried in an oven (UF 55, Memmert, USA).
Characterization of TFC membrane
Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy. Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy was used to analyse the functional groups on the membrane surface and to examine the effectiveness of the interfacial polymerization. The ATR-FTIR spectra were obtained using a Thermo Scientific Nicolet iS-10 Fourier Transform Infrared (FTIR) spectrometer equipped with Attenuated Total Reflection (ATR) element of Smart iTX AR Diamond and an Omnic 5.1 software. The membrane active layers were pressed tightly against the crystal plate. The TFC membrane, PES membrane, TEOA and TMC were analysed, with each spectrum averaged from 256 scans that were collected from 650 to 4000 cm−1 at 2 cm−1 resolution. The analysis was performed with air as the background and no baseline or ATR corrections were applied.
Field emission scanning electron microscope (FESEM). The membrane was examined by a field emission scanning electron microscope (FESEM, JEOL Model JSM-6330F). The membranes were then placed adequately over a support and coated with platinum under vacuum conditions to examine the surface. The surface of membranes was observed under 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification.
000× magnification.
Pure water permeability (PWP). The value of PWP was experimentally determined by plotting water flux against applied pressure with the assumption of null value of osmotic pressure. PWP experiment was performed by measuring the time taken for 20 mL of ultrapure water collected at different pressure (2, 3, and 4 bar) constant stirring speed of 300 rpm using nanofiltration set-up mentioned in following section.
Approximate characterization method using uncharged solute. Previous studies26,27 have shown the effective pore radius (rp), and the ratio of effective membrane thickness over porosity for TFC nanofiltration membranes are best characterized using approximate characterization method. The flux and rejection data was fitted using the Donnan-Steric-Pore Model (DSPM) and Hagen–Poiseuille equation.The DSPM model is based on the extended Nernst–Planck equation expressed below. The model gives the flux of the solute i (ji) resulting from transport due to diffusion, electrical and convection forces.
| |
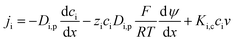 | (1) |
where,
For uncharged solute, the transport is governed purely by diffusive and convective flows inside the membrane thereby reducing the above mentioned Nernst–Planck equation to
| |
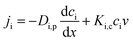 | (3) |
Ki,d and
Ki,c which are functions of the ratio of solute to pore radius (
λ), account for the hindrance due to diffusion and convection, respectively.
Ki,d and
Ki,c can be related to the hydrodynamic coefficients
K−1 (enhanced drag) and
G (lag coefficient) according to the following equations:
| | |
Ki,d = K−1(λ,0) = 1.0 − 2.30λ + 1.154λ2 + 0.224λ3
| (4) |
| | |
Ki,c = G(λ,0) = (2 − ϕ)(1.0 + 0.054λ − 0.988λ2 + 0.441λ3)
| (5) |
where,
| |
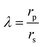 | (6) |
λ is the ratio of solute radius (
rs) to pore size (
rp) where,
λ has the limitation of 0 <
λ < 0.95.
28 Ø is the steric terms relating the finite size of the solute and pore size according to the following equation,
In term of real rejection (Rreal), eqn (3) becomes
| |
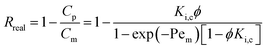 | (8) |
where
Cp the concentration of solute in the permeate, and
Cm the concentration of solute on the membrane. The Peclet number (Pe
m) is defined as,
| |
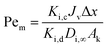 | (9) |
where
Di,∞ is the bulk diffusivity of solute (m
2 s
−1),
Jv the volume flux (based on membrane area) (m s
−1), and Δ
x/
Ak the ratio of effective membrane thickness over porosity. The Hagen–Poiseuille equation relates the pure water flux and the applied pressure across the membrane,
| |
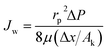 | (10) |
where,
Jw is the water flux (m
3 m
−2 s
−1), Δ
P is the applied transmembrane pressure (Pa) and
μ is the viscosity of the solution (Pa s). Concentration polarization equation was employed to find the concentration of solute on the membrane surface,
Cm. For a stirred cell configuration,
26 the observed rejection (
Robs) was related to the real rejection by volume flux,
Jv and mass transfer coefficient,
k in
eqn (11) and
(12).
| |
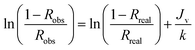 | (11) |
| |
 | (12) |
where,
rr is the radius of effective membrane area (m),
ν the kinematic viscosity (m
2 s
−1), and
ω the stirring speed (rad s
−1).
Table 1 list all the properties needed for calculation. The diffusivity data and Strokes radius were referred from published works.
8
Table 1 Physical properties of monosaccharides and water
| Property |
Value |
| rr |
2.68 × 10−2 m |
| ω |
31.41 rad s−1 |
| ν at 25 °C |
8.94 × 10−4 m2 s−1 |
| μ at 25 °C |
8.94 × 10−04 Pa s |
| D∞,xylose |
7.50 × 10−10 m2 s−1 |
| D∞,glucose |
6.73 × 10−10 m2 s−1 |
| rs,xylose (Strokes radius) |
3.25 × 10−10 m |
| rs,glucose (Strokes radius) |
3.65 × 10−10 m |
| Molecular weight xylose |
150.30 g mol−1 |
| Molecular weight glucose |
180.60 g mol−1 |
Contact angle measurement. The contact angles of all the membranes were measured using contact angle goniometer (JY-82, Chengde testing machine Co. Ltd., China). A dangling droplet of 5 μL of ultrapure water at the end of ‘I’-shaped needle was carefully deposited to membrane surface to avoid the effect of falling force by gravity. A static image of the droplet in equilibration with the membrane surface was taken. For ensuring the accuracy, the measurements were performed at 5 different locations, and then the average value was regard as the final contact angle result.
Nanofiltration experiments
Nanofiltration experiments have been carried out using the stirred cell system schematized in Fig. 1. A Millipore stirred cell (Model 8200, Millipore-Amicon Corporation, USA) having a maximum volume uptake of 200 mL and an effective membrane area of 2.87 × 10−3 m2 was used in all experiments. Prepared TFC membrane was fitted into the membrane holder at the bottom of the stirred cell. Other parts are then assembled together and place on top of a magnetic stirrer (Model MS-20D, Daihan Scientific Co. Ltd., South Korea). The xylose–glucose mixture solution for nanofiltration were prepared by dissolving 5 g of xylose and 5 g of glucose in 1 L of deionized water. This mixture gives concentration of 5 g L−1 of xylose and 5 g L−1 of glucose. 180 mL of the xylose–glucose mixture solution was filled into the stirred cell. The nanofiltration was performed by collecting 20 mL of permeate at pressure 4 bar and stirring speed of 300 rpm at room temperature. After the nanofiltration, the composite membrane was flushed by deionized water at a stirring speed of 350 rpm for 30 minutes, without applying any pressure.
 |
| | Fig. 1 Schematic diagram of nanofiltration system. | |
Quantification of sugar using high performance liquid chromatography (HPLC)
Quantification of samples was done using HPLC (1200 Series, Agilent Technologies, USA) equipped with refractive index detector. The liquid chromatography column used in this study was Agilent's Zorbax Carbohydrate 5 μm (4.6 × 250 mm) with an operating flow rate of 1 mL min−1 and injection volume of 20 μL. The mobile phase used during analysis was prepared by diluting three parts for acetonitrile with one part of ultrapure water. Prior to HPLC analysis, the mobile phase was filtered using nylon membrane with pore size of 0.22 μm and degassed using ultrasonic bath at room temperature for 1 hour. All HPLC samples were filtered using a 3 mL syringe with a 0.2 μm filter attached.
Calculations for separation evaluation
The performance of membrane was measured using xylose separation factor. The xylose separation factor (Xxyl), is a measure of xylose purification from glucose calculated using eqn (13), slightly modified from Sjoman et al.8 This factor indicates the change in the permeate composition compared to the original ratio of xylose to glucose in the bulk. A value of one implies that no separation between xylose and glucose occurs. While, value greater than one implies ratio of xylose over glucose is higher in permeate than the original ratio (feed), thus xylose enriched in permeate. Value lower than one implies the opposite, where glucose is enriched rather than xylose.| |
 | (13) |
where,| |
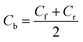 | (14) |
| |
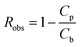 | (15) |
Cp_xyl is the concentration of xylose in permeate (g/100 g of solution), Cb_xyl is the concentration of xylose in the bulk (g/100 g of solution), Cp_glu is the concentration of glucose in permeate (g/100 g of solution), Cb_glu is the concentration of glucose in the bulk (g/100 g of solution), Cb the concentration of respective solute in bulk (g/100 g of solution), Cf the concentration of respective solute in feed (g/100 g of solution), Cr the concentration of respective solute in retentate (g/100 g of solution), Cp the concentration of respective solute in permeate (g/100 g of solution), and Robs the observed retention of respective solute. The observed retention, Robs is a measure of membrane selectivity toward a solute.
Design of experiment
Experimental designs were carried out using Design Expert version 7.0.0 (Stat-Ease Inc., USA). Fractional factorial designs were applied in this study to reserve some resources for unforeseen contingencies and follow-up runs.24 A 25−1 fractional factorial design were used to analyse the statistical significance of each factors influencing separation performance, and consequently, this design included 16 experimental runs. Xylose separation factor was taken as the response or output variable of the factorial design experiments. Five independent variables considered for the factorial design were the concentration of TEOA (A), the concentration of TMC (B), reaction time (C), pH of the aqueous solution (D), and curing (E). All the factors studied in this were numerical factors except curing which is a categorical factor. Each variable was examined at a high (coded +1) and low (coded −1) level. Table 2 showed the independent variables for screening process using fractional factorial design. The low level for curing factor was determined to be “No” where the membrane is left dried at room temperature. Meanwhile, curing at the high level “Yes” is where membrane is dried inside an oven (UF 55, Memmert, USA) at 60 °C for 30 minutes.
Table 2 Interfacial polymerization parameters in the factorial design
| Factor |
Units |
Low value (−1) |
High value (+1) |
| A: concentration of triethanolamine (TEOA) |
% wt |
4% |
8% |
| B: concentration of tri-mesoyl chloride (TMC) |
% wt |
0.05% |
0.25% |
| C: reaction time |
Minutes |
25 |
45 |
| D: pH of aqueous solution |
|
8 |
12 |
| E: curing |
|
No |
Yes |
Results and discussion
Interfacial polymerization
The TFC membrane used for characterization purposes was prepared at TEOA concentration of 4% (w/v), TMC concentration of 0.25% (w/v), the reaction time of 45 minutes, and aqueous solution pH at 8, and cured at a temperature of 60 °C. The membranes were characterized using ATR-FTIR spectroscopy to analyze the surface chemistry of PES membrane before and after IP between TEOA and TMC. Fig. 2 showed the spectra belonging to virgin PES membrane and freshly prepared TFC membrane. A strong peak at wavenumber 1724 cm−1 was seen in the spectra of TFC membrane beside the typical PES bands. This peak falls into the band belonging to a functional group of ester (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch),15,29 similar to two out of three ideal categories of cross-linked polymer chains formed from the reaction between TEOA and TMC proposed by Tang et al.15 The typical PES bands in TFC membrane spectra have weaker peaks than the one in PES spectra as seen in Fig. 2. An infrared beam of ATR-FTIR penetrates the membrane to a certain depth. With the addition of a layer of polymer on top of the support membrane, the absorbance of infrared beam by the support membrane became lesser at the same depth. This verified formation of thin-film on top of the PES membrane.
O stretch),15,29 similar to two out of three ideal categories of cross-linked polymer chains formed from the reaction between TEOA and TMC proposed by Tang et al.15 The typical PES bands in TFC membrane spectra have weaker peaks than the one in PES spectra as seen in Fig. 2. An infrared beam of ATR-FTIR penetrates the membrane to a certain depth. With the addition of a layer of polymer on top of the support membrane, the absorbance of infrared beam by the support membrane became lesser at the same depth. This verified formation of thin-film on top of the PES membrane.
 |
| | Fig. 2 ATR-FTIR spectra for PES membrane and TFC membrane. | |
Fig. 3 showed the FESEM images of surface of both, support PES membrane and TFC membrane at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification. The support PES membrane in Fig. 3(i) has a smooth surface with porous structure while TFC membrane in Fig. 3(ii) has a rough surface with nonporous structure. This distinct different of surface roughness and porosity proved that there was the formation of TFC layer on top of the support PES membrane by IP. The contact angle for support PES membrane was 51.03 ± 3.68° and TFC membrane was 30.08 ± 1.27° as shown in Fig. 4. TFC membrane has smaller contact angle than the PES implying that formation of polyester increases the hydrophilicity of the membrane. The increase in membrane hydrophilicity does favours retention of sugars.16 Past study found that the commercial nanofiltration membranes had PWP between 1.33 and 50.50 L m−2 h−1 bar−1.27 From this study, the membranes prepared had PWP between 0.72 and 33 L m−2 h−1 bar−1 as shown in Table 3, which is within the commercial nanofiltration range as described in the past study.27 The PWP of PES support membrane was 59.54 L m−2 h−1 bar−1, which were not within the nanofiltration PWP. By comparing the PWP between PES support membrane and TFC membrane, there is a notable reduction in PWP of the TFC membrane from the PES support membrane around 44.6% to 98.8%. Again, reduction in PWP was another prove of TFC formation on top of the support membrane.
000× magnification. The support PES membrane in Fig. 3(i) has a smooth surface with porous structure while TFC membrane in Fig. 3(ii) has a rough surface with nonporous structure. This distinct different of surface roughness and porosity proved that there was the formation of TFC layer on top of the support PES membrane by IP. The contact angle for support PES membrane was 51.03 ± 3.68° and TFC membrane was 30.08 ± 1.27° as shown in Fig. 4. TFC membrane has smaller contact angle than the PES implying that formation of polyester increases the hydrophilicity of the membrane. The increase in membrane hydrophilicity does favours retention of sugars.16 Past study found that the commercial nanofiltration membranes had PWP between 1.33 and 50.50 L m−2 h−1 bar−1.27 From this study, the membranes prepared had PWP between 0.72 and 33 L m−2 h−1 bar−1 as shown in Table 3, which is within the commercial nanofiltration range as described in the past study.27 The PWP of PES support membrane was 59.54 L m−2 h−1 bar−1, which were not within the nanofiltration PWP. By comparing the PWP between PES support membrane and TFC membrane, there is a notable reduction in PWP of the TFC membrane from the PES support membrane around 44.6% to 98.8%. Again, reduction in PWP was another prove of TFC formation on top of the support membrane.
 |
| | Fig. 3 FESEM surface images of (i) PES membrane and (ii) TFC membrane at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification. 000× magnification. | |
 |
| | Fig. 4 Contact angle measurement for PES membrane and TFC membrane. | |
Table 3 Response and characterization of TFC membrane
| Std |
Coded variables |
Response and characterization |
| A: conc. TEOA% (w/v) |
B: conc. TMC% (w/v) |
C: reaction time minutes |
D: pH |
E: curing |
Xxyl |
PWP L m−2 h−1 bar−1 |
Avg. rp nm |
Avg. Δx/Ak μm |
| 1 |
−1 |
−1 |
−1 |
−1 |
+1 |
1.178 |
6.66 |
0.370 |
1.04 |
| 2 |
+1 |
−1 |
−1 |
−1 |
−1 |
0.966 |
33.01 |
0.363 |
0.20 |
| 3 |
−1 |
+1 |
−1 |
−1 |
−1 |
0.953 |
2.27 |
0.438 |
4.26 |
| 4 |
+1 |
+1 |
−1 |
−1 |
+1 |
1.283 |
2.37 |
0.423 |
3.80 |
| 5 |
−1 |
−1 |
+1 |
−1 |
−1 |
1.013 |
12.37 |
0.363 |
0.54 |
| 6 |
+1 |
−1 |
+1 |
−1 |
+1 |
1.507 |
6.30 |
0.375 |
1.13 |
| 7 |
−1 |
+1 |
+1 |
−1 |
+1 |
1.616 |
0.72 |
0.428 |
12.86 |
| 8 |
+1 |
+1 |
+1 |
−1 |
−1 |
1.036 |
2.27 |
0.421 |
3.93 |
| 9 |
−1 |
−1 |
−1 |
+1 |
−1 |
1.049 |
18.89 |
0.363 |
0.35 |
| 10 |
+1 |
−1 |
−1 |
+1 |
+1 |
1.163 |
15.65 |
0.363 |
0.43 |
| 11 |
−1 |
+1 |
−1 |
+1 |
+1 |
1.278 |
0.85 |
0.433 |
11.14 |
| 12 |
+1 |
+1 |
−1 |
+1 |
−1 |
0.807 |
1.71 |
0.432 |
5.52 |
| 13 |
−1 |
−1 |
+1 |
+1 |
+1 |
0.882 |
6.50 |
0.376 |
1.10 |
| 14 |
+1 |
−1 |
+1 |
+1 |
−1 |
1.187 |
18.54 |
0.363 |
0.36 |
| 15 |
−1 |
+1 |
+1 |
+1 |
−1 |
1.472 |
1.56 |
0.433 |
6.03 |
| 16 |
+1 |
+1 |
+1 |
+1 |
+1 |
0.969 |
1.36 |
0.431 |
6.88 |
The rp and Δx/Ak of the prepared membranes calculated using approximate characterization method and summarized in Table 3. The calculated average rp ranged from 0.363 nm to 0.438 nm and Δx/Ak from 0.2 μm to 12.86 μm. The average rp and Δx/Ak of all prepared membranes showed that all the TFC membrane prepared were within commercial NF range, in agreement with the past study.27 Commonly, it is believed that sieving effect is the main separation mechanism in separating xylose and glucose using NF membrane.8 Observation on Table 3 showed that larger rp of developed TFC membranes has better xylose separation factor with some of their rp larger than the equivalent molar radius of xylose and glucose, which are 0.34 nm and 0.36 nm, respectively. The lack of correlation between xylose separation factor and average rp as shown in Table 3 does not reflect the sieving effect separation mechanism. This showed that separation of xylose and glucose may not only govern by one separation mechanism. Retention of uncharged solute in past studies do affected by diffusion mechanism,30 and hydrophobicity of the compound.16
Model fitting
Simulation and analysis of experimental data by a complete 16 fractional factorial design was systematically conducted to calculate effect estimates using Yates algorithms. The percent contribution comes from adding up the total sum of squares and then taking each term's sum of squares and dividing by the total to get a percentage.31 The effect estimate and percent contribution was calculated and tabled in Table 4. The selection of interaction terms for the model were based on the percent contribution, however main effects bypass this process due to model hierarchy. Interaction terms with percent contribution more than 1% were chosen for the regression model. The interaction terms were AB, AD, AE, BC, BE, CD, CE, and DE.
Table 4 Effect estimate and percent contribution for all model terms
| Term |
Effect estimate |
Sum of squares |
% contribution |
| A-conc. TEOA |
−0.065 |
0.017 |
2.081 |
| B-conc. TMC |
0.059 |
0.014 |
1.674 |
| C-reaction time |
0.126 |
0.063 |
7.685 |
| D-pH aqueous solution |
−0.093 |
0.035 |
4.223 |
| E-curing |
0.174 |
0.121 |
14.764 |
| AB |
−0.241 |
0.232 |
28.194 |
| AC |
−0.006 |
1.27 × 10−3 |
0.015 |
| AD |
−0.073 |
0.022 |
2.622 |
| AE |
0.057 |
0.013 |
1.603 |
| BC |
0.067 |
0.018 |
2.210 |
| BD |
0.003 |
2.76 × 10−5 |
0.003 |
| BE |
0.045 |
0.008 |
1.003 |
| CD |
−0.072 |
0.021 |
2.551 |
| CE |
−0.108 |
0.046 |
5.640 |
| DE |
−0.230 |
0.211 |
25.732 |
The relative size of effects are visually demonstrated as Pareto chart in Fig. 5, where the bar length are proportional to the absolute value of estimated effect. Effects of t-value limit (black line) are considered statistically significant at 95% confidence level while effects below t-value limit are not likely to be significant. Effect above Bonferroni's corrected t-value limit (red line) is almost certainly significant.31 A quick analysis was performed on the selected effects using Pareto chart to statistically check for significance of the selected effects at 95% confidence level. All the selected effects (A, B, C, D, E, AB, AD, AE, BC, BE, CD, CE, and DE) shown to be significant at both t-value limit and Bonferroni's corrected t-value limit.
 |
| | Fig. 5 Pareto chart of effects for response xylose separation factor. | |
The fitted model for the factorial analysis in coded form was shown in eqn (4). From this equation, the coefficients for all the selected terms are lower than the interception, which indicated the existent of the design plateau. Thus, these plateau showed that the design had an optimum point, where further optimization experiment can be performed.32
| |
 | (16) |
ANOVA
Table 5 summarizes the ANOVA results. A model is considered significant if its p-value is lower than 0.05, indicating only a 5% chance that a ‘Model F-value’ could occur because of noise. The p-value for the fitted model was 0.0012, the fitted model equation adequately describes the response. Coefficient of determination (R2) is the proportion of variation in the response attributed to the model. The R2 for the model was 0.9998, implying a high correlation between the observed and predicted values, as shown in Fig. 6.
| Source |
Sum of squares |
Degree of freedom |
Mean square |
F-Value |
p-Value |
|
| C.V. = 0.77%; R2 = 0.9998; adjusted R2 = 0.9986; Adeq. precision = 98.84. |
| Model |
0.821 |
13 |
0.063 |
820 |
0.0012 |
Significant |
| A-conc. TEOA |
0.017 |
1 |
0.017 |
222 |
0.0045 |
| B-conc. TMC |
0.014 |
1 |
0.014 |
178 |
0.0056 |
| C-reaction time |
0.063 |
1 |
0.063 |
819 |
0.0012 |
| D-pH aqueous solution |
0.035 |
1 |
0.035 |
450 |
0.0022 |
| E-curing |
0.121 |
1 |
0.121 |
1574 |
0.0006 |
| AB |
0.232 |
1 |
0.232 |
3005 |
0.0003 |
| AD |
0.022 |
1 |
0.022 |
279 |
0.0036 |
| AE |
0.013 |
1 |
0.013 |
171 |
0.0058 |
| BC |
0.018 |
1 |
0.018 |
236 |
0.0042 |
| BE |
0.008 |
1 |
0.008 |
107 |
0.0092 |
| CD |
0.021 |
1 |
0.021 |
272 |
0.0037 |
| CE |
0.046 |
1 |
0.046 |
601 |
0.0017 |
| DE |
0.211 |
1 |
0.211 |
2743 |
0.0004 |
| Residual |
1.54 × 10−4 |
2 |
7.71 × 10−5 |
|
|
| Cor total |
0.821 |
15 |
|
|
|
 |
| | Fig. 6 Predicted vs. actual xylose separation factor. | |
Best condition and model validation
Numerical optimization was performed on the fitted model with the goal to maximize the response. The optimization gave the following best conditions as shown in Table 6 with predicted response for xylose separation factor at 1.614 at desirability of 0.0.995. The suitability of the model equation for predicting the optimum response value was tested under the best conditions as described in Table 6. The validation experiments were conducted at the suggested best conditions and the error from these runs were 4.6%, 5.7%, and 4.3% as shown in Table 7. Based on the predicted and experimental results presented, the experimental values were in good agreement with the predicted values proposed by the model with an error less than 10% and proved to be an adequate model.
Table 6 Suggested best condition for factors
| Factors |
Best condition |
| A-conc. TEOA |
4% (w/v) |
| B-conc. TMC |
0.25% (w/v) |
| C-reaction time |
44.85 minutes |
| D-pH aqueous solution |
8 |
| E-curing |
Yes |
Table 7 Comparison between predicted and experimental value for optimum condition
| Description |
Xylose separation factor |
| Run 1 |
Run 2 |
Run 3 |
| Predicted value |
1.614 |
1.614 |
1.614 |
| Experimental value |
1.691 |
1.527 |
1.686 |
| Error |
4.7% |
5.7% |
4.3% |
The membrane properties and performances of commercial (Desal-5 DL, Desal-5 DK, and NF270), and TFC membranes developed at its best conditions in this work were summarized in Table 8 for comparison purposes. Also listed in Table 8 were characterizations of self-made membranes prepared at its best conditions. It should also be noted, comparison were made relatively because of the different operating parameters, especially pressure and temperature between this work and previous ones. The PWP at the same TEOA concentration (4% wt), and contact angle obtained in this work were comparable with Tang et al.,15,33 with membranes in this work having slightly better surface hydrophilicity and PWP. A brief comparison between this work and other self-made membranes also revealed, this work has comparable performance in term of PWP but has lower membrane surface hydrophilicity. The developed membrane in this work also has slightly larger pore size than the others. In general, the developed TFC membrane in this study have common ground with other self-made TFC membrane developed in the past. The hydrophilicity of TFC membranes developed in this work were also comparable to the other commercial membranes, except Desal-5 DL being the least hydrophilic membrane. An interesting trend was found when relating hydrophilicity of membranes to xylose separation factor in Table 8. Membranes with more hydrophilicity has better separation performance, showing hydrophilicity plays some role in separating xylose from glucose in addition to membrane pore size. The PWP of developed TFC membranes were the lowest compared to the others, although having similar hydrophilicity with Desal-5 DK and NF270. Typically, PWP of hydrophilic membranes are higher than the hydrophobic membranes due to the hydrophilic–hydrophobic interaction between water with the membrane surface. However, this relationship were not seen in developed TFC membranes. Xylose and glucose rejections obtained by developed TFC membranes were surprisingly higher than commercial ones, suggesting more solutes were retained. Taking both PWP and rejections data into consideration, developed TFC membranes significantly hindered the transport of solute and solvent through the membranes suggesting developed TFC membranes have denser skin layer and tighter pores than the commercial ones. Xylose separation factor obtained by TFC membranes prepared under best conditions in this work were comparable to commercial membranes, like Desal-5 DL, Desal-5 DK, and NF270. This showed tailoring TFC membrane for separating xylose from glucose can be done by controlling the properties of thin-layer by manipulating the preparative parameters.
Table 8 Comparison of the membrane properties and performances of commercial and TFC membranes developed in this work at the best conditions
| Membrane |
Contact angle (°) |
PWPa (L m−2 h−1 bar−1) |
Pore size (nm) |
Robs_xyl |
Robs_glu |
Xxyl |
Taken from observed rejections of xylose and glucose with 2% wt monosaccharide solution, mass ratio of xylose to glucose in the feed solution at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, temperature at 50 °C, and applied pressure of 5 bar.8 The feed contains 1% wt monosaccharide solution, mass ratio of xylose to glucose in the feed solution at 1 1, temperature at 50 °C, and applied pressure of 5 bar.8 The feed contains 1% wt monosaccharide solution, mass ratio of xylose to glucose in the feed solution at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, temperature of 25 °C, and pressure applied was 4 bar. 1, temperature of 25 °C, and pressure applied was 4 bar. |
| Desal-5 DL |
41,34 44,35 54.4 (ref. 36) |
8.1 ± 1.5 (ref. 8) |
0.50 (ref. 37) |
0.19a |
0.45a |
1.47 |
| Desal-5 DK |
31 (ref. 38) |
9.1 ± 1.9 (ref. 8) |
0.58–0.67 (ref. 39) |
0.59a |
0.85a |
2.73 |
| NF270 |
26,36 30 (ref. 38) |
15.9 ± 1.1 (ref. 8) |
0.43,37 0.39–0.44 (ref. 40) |
0.29a |
0.55a |
1.58 |
| Other self-made membranes |
35,33 12–25,41 15–20,42 |
7.4,41 13.2,42 1.3,15 1.7,43 10.3,44 3.1 (ref. 45) |
0.33–0.42,41 0.42 (ref. 42) |
— |
— |
— |
| This work at best conditions |
30 |
2.0 ± 0.5 |
0.47 |
0.83b |
0.90b |
1.64 |
Effect of interfacial polymerization factors on xylose separation factor
The positive effects were colored in orange and the negative ones in blue in the Pareto chart (Fig. 5). For main effects, an effect is said to be positive when an increase to its high level will cause an increase in the response, while negative effect is when an increase to its high level will result a decrease in the response. For interaction between main effects, positive effect is when both factors were change to the same level (low or high), it will increase the response. Negative effect is when both factors were change to the opposite level (one at its low and the other at its high), the response will increase.46
Factor A and D are found to have negative effect, whereas factor B, C, and E are in positive effect. It can be seen from Fig. 5 that factor A and B are the two lowest main effects on xylose separation factor. Factor A was described in past study to be the main factor in determining the reaction rate between TEOA and TMC since TEOA has low reaction activity.15 While, factor B was reported to be the important factor in determining the reaction rate since interfacial polymerization is a diffusion controlled process in the organic layer.23 In this study, both factors (A and B) are found to be less effective on xylose separation factor when alone (individual main effects). However the interaction between these two factors has the highest effect on xylose separation factor. According to Fig. 7(i), xylose separation factor was improved when TMC concentration were increased from 0.05% (w/v) to 0.25% (w/v) at TEOA concentration of 4% (w/v). At TEOA concentration of 8% (w/v), xylose separation factor decreased when TMC concentration were increased from 0.05% (w/v) to 0.25% (w/v). In term of polymerization, this is true since both are monomers required for polycondensation to occur. If one of the monomer is the excess reactant, the other monomer will be the limiting reactant. Hence, both monomers can be the reaction rate determining factor, given one of the monomer is in excess.
 |
| | Fig. 7 Interaction effects plots for (i) TEOA and TMC concentration, (ii) TEOA concentration and pH aqueous solution, (iii) reaction time and curing, and (iv) pH aqueous solution and curing. | |
Factor D was also found to have less influence on xylose separation factor when alone but gave a high influence when interact with factor E. Both factors D and E are found to be affecting separation performance but no correlation was found since past study47 used OFAT design. It could miss out the interaction between factor D and E in enhancing separation. TFC membrane exposed to curing has significantly better xylose separation factor at pH 8 as in Fig. 7(iv). However, further increased to pH 12 resulted in no improvement toward xylose separation factor. Factor D alone was reported by Tang et al.15 to give an impact on enhancing separation where hydrogen chloride, a by-product during interfacial polymerization will be neutralized by the sodium hydroxide in aqueous solution. Thus, a dense TFC layer will form on top of support membrane through the gradual formation of polymer with high molecular weight from the positive reaction of chemical equilibrium. Further investigation is needed in order to explain the correlation between these two factors which could greatly enhance separation. It is postulated that the crystallization rate and amount of salt from the neutralization of hydrogen chloride during the curing period may have some influence on the pore structure hence the separation performance. Sodium hydroxide in aqueous solution with the higher pH may neutralized more hydrogen chloride followed by high crystallization rate of salt in high temperature, resulted in damaging the pores of membrane by formation of large salt crystal.
Factor C and E were found to be independent with each other because their interaction effect have lesser influence on xylose separation factor compared to their individual main effects. Reaction time (C) determine the extent of reaction governing the formation of TFC layer, which later affect the performance of separation.23 In this study, having longer reaction time was more beneficial because it is statistically proven in Fig. 5 where factor C has huge positive effect on xylose separation factor. Moreover, the positive effect of factor E on xylose separation factor suggested that curing in temperature of 60 °C is necessary for formation of better TFC membrane top layer. This was illustrate by the interaction between TMC concentration and curing in Fig. 7(iii). Curing at higher temperature rapidly evaporate excess surface organic solvent allowing more cross-linking to occurs, hence the improvement on separation performance.48 Another interesting interaction were seen between TEOA concentration and pH of aqueous solution as plotted in Fig. 7(ii). At TEOA concentration of 4% (w/v), increase in pH of aqueous solution drastically improve the performance of xylose separation. This can related to the neutralization of hydrogen chloride produce during polymerization between TEOA and TMC. The neutralization of hydrogen chloride is important to avoid the formation of salts or soaps derived between TEOA and hydrogen chloride which reduce the amount of TEOA available for crosslinking with TMC.
Based on the magnitude of the individual effects, the ranking of factors in preparation of TFC membrane for separation of xylose from glucose were curing > reaction time > pH of aqueous solution > TEOA concentration > TMC concentration. From these factors, curing, reaction time and TMC concentration have positive effects on xylose separation factor within the design space studied in this work. Although all three were significant at 95% confidence interval, only curing and reaction time above Bonferroni's corrected t-value limit shown in Fig. 5. This come to the conclusion that curing and reaction time were the significant factors in developing TFC membrane for xylose separation within the range of factors studied in this work.
Conclusions
TFC membrane developed from IP of TEOA and TMC on PES membrane has shown the ability in separating xylose from glucose. The characterization by using ATR-FTIR, FESEM, contact angle, and pure water permeability showed that a rough and nonporous polyester layer was formed on the surface of the PES supporting membrane.
In this study, a 25−1 fractional factorial design was used to evaluate the significant factors in preparation of TFC membrane for separation of xylose from glucose. Five factors include TEOA concentration, TMC concentration, reaction time, pH of aqueous solution, and curing were investigated as design variables and xylose separation factor was considered as experimental design response. The ranking of factors in preparation of TFC membrane for separation of xylose from glucose were curing > reaction time > pH of aqueous solution > TEOA concentration > TMC concentration. From these factors, curing, reaction time and TMC concentration have positive effects on xylose separation factor within the design space studied in this work. The model in this work will be useful for further optimization by response surface methodology in order to create a second-order model, which can predict the responses more accurately. Manipulation of preparative parameters using fractional factorial design to narrow down significant factors not only hasten the tailoring process, but also reduce the usage of resources compared to the traditional OFAT design.
Although the achieved separation factor was not as high as commercial membranes cited in the literature, this study demonstrated the ability to specifically tailored composite membrane for xylose–glucose separation using IP. The discrepancy in separation performance may due to the huge difference in operating parameters, such as pressure, total sugar concentration, temperature, and ratio between xylose and glucose. The membranes in this work were tested at the same nanofiltration parameters. Investigation and optimization on nanofiltration parameters for membranes in this work may further improve separation factor and expand the application of this work. This is because possible changes in the membrane structure, like compaction and swelling, due to pressure, filtration temperature and pH of solution might have an influence on the separation.
Acknowledgements
The authors wish to express their thanks to Ministry of Education Malaysia through the financial aid from grant GRS1403113, RDU140901 and LRGS/2013/UKM-UKM/PT/03. The authors also wish to acknowledge the Ministry of Education Malaysia for sponsoring K. H. Mah postgraduate's study via MyBrain.
Notes and references
- J. E. Almazan, E. M. Romero-Dondiz, V. B. Rajal and E. F. Castro-Vidaurre, Chem. Eng. Res. Des., 2015, 94, 485–493 CrossRef CAS.
- M. A. K. M. Zahari, S. S. S. Abdullah, A. M. Roslan, H. Ariffin, Y. Shirai and M. A. Hassan, J. Cleaner Prod., 2014, 65, 252–260 CrossRef CAS.
- I. Roberto, S. Mussatto and R. Rodrigues, Ind. Crops Prod., 2003, 17, 171–176 CrossRef CAS.
- O. Hassan, T. P. Ling, M. Y. Maskat, R. M. Illias, K. Badri, J. Jahim and N. M. Mahadi, Biomass Bioenergy, 2013, 56, 137–146 CrossRef CAS.
- C. Ofori-Boateng, K. T. Lee and B. Saad, Energy Convers. Manage., 2014, 81, 192–200 CrossRef CAS.
- Y. M. Feng, X. L. Chang, W. H. Wang and R. Y. Ma, J. Taiwan Inst. Chem. Eng., 2009, 40, 326–332 CrossRef CAS.
- S. S. Da Silva and A. K. Chandel, D-Xylitol: Fermentative Production, Application, and Commercialization, Springer, Heidelberg, 2012 Search PubMed.
- E. Sjoman, M. Manttari, M. Nystrom, H. Koivikko and H. Heikkila, J. Membr. Sci., 2007, 292, 106–115 CrossRef.
- A. K. Goulas, P. G. Kapasakalidis, H. R. Sinclair, R. A. Rastall and A. S. Grandison, J. Membr. Sci., 2002, 209, 321–335 CrossRef CAS.
- L. Moreno-Vilet, J. Bonnin-Paris, S. Bostyn, M. A. Ruiz-Cabrera and M. Moscosa-Santillán, Sep. Purif. Technol., 2014, 131, 84–93 CrossRef CAS.
- S. T. Morthensen, J. Luo, A. S. Meyer, H. Jørgensen and M. Pinelo, J. Membr. Sci., 2015, 492, 107–115 CrossRef CAS.
- W. J. Lau, A. F. Ismail, N. Misdan and M. A. Kassim, Desalination, 2012, 287, 190–199 CrossRef CAS.
- W. J. Lau and A. F. Ismail, in 2nd International Conference on Environmental Engineering and Application, 2011, pp. 173–177 Search PubMed.
- N. Hilal, M. Khayet and C. J. Wright, Membrane modification: Technology and applications, CRC Press, Taylor & Francis Group, Florida, USA, 2012 Search PubMed.
- B. Tang, Z. Huo and P. Wu, J. Membr. Sci., 2008, 320, 198–205 CrossRef CAS.
- L. Braeken, R. Ramaekers, Y. Zhang, G. Maes, B. Van Der Bruggen and C. Vandecasteele, J. Membr. Sci., 2005, 252, 195–203 CrossRef CAS.
- A. K. Ghosh and E. M. V. Hoek, J. Membr. Sci., 2009, 336, 140–148 CrossRef CAS.
- A. F. Ismail, M. Padaki, N. Hilal, T. Matsuura and W. J. Lau, Desalination, 2015, 356, 140–148 CrossRef CAS.
- B. S. Lalia, V. Kochkodan, R. Hashaikeh and N. Hilal, Desalination, 2013, 326, 77–95 CrossRef CAS.
- R. J. Petersen, J. Membr. Sci., 1993, 83, 81–150 CrossRef CAS.
- N. K. Saha and S. V. Joshi, J. Membr. Sci., 2009, 342, 60–69 CrossRef CAS.
- N. A. Jalanni, M. N. Abu Seman and C. K. M. Faizal, Jurnal Teknologi, 2013, 65, 69–72 CrossRef.
- A. L. Ahmad and B. Ooi, J. Membr. Sci., 2005, 255, 67–77 CrossRef CAS.
- J. Telford, Johns Hopkins APL Tech. Dig., 2007, 27, 224–232 Search PubMed.
- R. Mee, A comprehensive guide to factorial two-level experimentation, Springer, London, 2009 Search PubMed.
- W. Bowen, A. W. Mohammad and N. Hilal, J. Membr. Sci., 1997, 126, 91–105 CrossRef CAS.
- W. Bowen and A. A. W. Mohammad, Chem. Eng. Res. Des., 1998, 76, 885–893 CrossRef CAS.
- W. Bowen and A. O. Sharif, J. Colloid Interface Sci., 1994, 168, 414–421 CrossRef CAS.
- M. N. Abu Seman, M. Khayet and N. Hilal, J. Membr. Sci., 2010, 348, 109–116 CrossRef CAS.
- B. Van Der Bruggen and C. Vandecasteele, Water Res., 2002, 36, 1360–1368 CrossRef CAS PubMed.
- M. J. Anderson, P. J. Whitcomb, S. L. Kraber and W. Adams, Stat-Ease Handbook for Experimenters, Stat-Ease, Inc, Minneapolis, 2009 Search PubMed.
- G. E. P. Box, W. J. Hunter and J. S. Hunter, Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building, Wiley, 1978, vol. 1 Search PubMed.
- B. Tang, C. Zou and P. Wu, J. Membr. Sci., 2010, 365, 276–285 CrossRef CAS.
- M. Mänttäri, A. Pihlajamäki and M. Nyström, J. Membr. Sci., 2006, 280, 311–320 CrossRef.
- K. Boussu, B. Van der Bruggen, A. Volodin, J. Snauwaert, C. Van Haesendonck and C. Vandecasteele, J. Colloid Interface Sci., 2005, 286, 632–638 CrossRef CAS PubMed.
- G. Cornelis, K. Boussu, B. Van Der Bruggen, I. Devreese and C. Vandecasteele, Ind. Eng. Chem. Res., 2005, 44, 7652–7658 CrossRef CAS.
- J. Luo and Y. Wan, J. Membr. Sci., 2011, 372, 145–153 CrossRef CAS.
- J. Tanninen, S. Platt, A. Weis and M. Nystr, J. Membr. Sci., 2004, 240, 11–18 CrossRef CAS.
- N. Ben Amar, H. Saidani, J. Palmeri and A. Deratani, Desalination, 2009, 246, 294–303 CrossRef CAS.
- H. Q. Dang, W. E. Price and L. D. Nghiem, Sep. Purif. Technol., 2014, 125, 43–51 CrossRef CAS.
- G.-E. Chen, Y.-J. Liu, Z.-L. Xu, Y.-J. Tang, H.-H. Huang and L. Sun, RSC Adv., 2015, 5, 40742–40752 RSC.
- X. Q. Cheng, L. Shao and C. H. Lau, J. Membr. Sci., 2015, 476, 95–104 CrossRef CAS.
- D. Hu, Z. Xu, Y. Wei, S. Cao and W. Chen, Sep. Sci. Technol., 2012, 48, 554–563 CrossRef.
- L. Li, S. Zhang and X. Zhang, J. Membr. Sci., 2009, 335, 133–139 CrossRef CAS.
- L. Shao, X. Q. Cheng, Y. Liu, S. Quan, J. Ma, S. Z. Zhao and K. Y. Wang, J. Membr. Sci., 2013, 430, 96–105 CrossRef CAS.
- E. Martendal, D. Budziak and E. Carasek, J. Chromatogr. A, 2007, 1148, 131–136 CrossRef CAS PubMed.
- C. Zhou, Y. Shi, C. Sun, S. Yu, M. Liu and C. Gao, J. Membr. Sci., 2014, 471, 381–391 CrossRef CAS.
- A. K. Ghosh, B.-H. Jeong, X. Huang and E. M. V. Hoek, J. Membr. Sci., 2008, 311, 34–45 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  a,
H. W. Yussof*a,
M. N. Abu Seman
a,
H. W. Yussof*a,
M. N. Abu Seman a and
A. W. Mohammadb
a and
A. W. Mohammadb
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification.
000× magnification.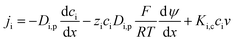
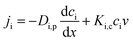
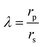
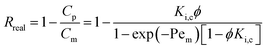
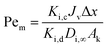
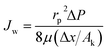
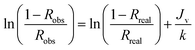


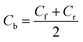
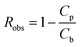
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch),15,29 similar to two out of three ideal categories of cross-linked polymer chains formed from the reaction between TEOA and TMC proposed by Tang et al.15 The typical PES bands in TFC membrane spectra have weaker peaks than the one in PES spectra as seen in Fig. 2. An infrared beam of ATR-FTIR penetrates the membrane to a certain depth. With the addition of a layer of polymer on top of the support membrane, the absorbance of infrared beam by the support membrane became lesser at the same depth. This verified formation of thin-film on top of the PES membrane.
O stretch),15,29 similar to two out of three ideal categories of cross-linked polymer chains formed from the reaction between TEOA and TMC proposed by Tang et al.15 The typical PES bands in TFC membrane spectra have weaker peaks than the one in PES spectra as seen in Fig. 2. An infrared beam of ATR-FTIR penetrates the membrane to a certain depth. With the addition of a layer of polymer on top of the support membrane, the absorbance of infrared beam by the support membrane became lesser at the same depth. This verified formation of thin-film on top of the PES membrane.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification. The support PES membrane in Fig. 3(i) has a smooth surface with porous structure while TFC membrane in Fig. 3(ii) has a rough surface with nonporous structure. This distinct different of surface roughness and porosity proved that there was the formation of TFC layer on top of the support PES membrane by IP. The contact angle for support PES membrane was 51.03 ± 3.68° and TFC membrane was 30.08 ± 1.27° as shown in Fig. 4. TFC membrane has smaller contact angle than the PES implying that formation of polyester increases the hydrophilicity of the membrane. The increase in membrane hydrophilicity does favours retention of sugars.16 Past study found that the commercial nanofiltration membranes had PWP between 1.33 and 50.50 L m−2 h−1 bar−1.27 From this study, the membranes prepared had PWP between 0.72 and 33 L m−2 h−1 bar−1 as shown in Table 3, which is within the commercial nanofiltration range as described in the past study.27 The PWP of PES support membrane was 59.54 L m−2 h−1 bar−1, which were not within the nanofiltration PWP. By comparing the PWP between PES support membrane and TFC membrane, there is a notable reduction in PWP of the TFC membrane from the PES support membrane around 44.6% to 98.8%. Again, reduction in PWP was another prove of TFC formation on top of the support membrane.
000× magnification. The support PES membrane in Fig. 3(i) has a smooth surface with porous structure while TFC membrane in Fig. 3(ii) has a rough surface with nonporous structure. This distinct different of surface roughness and porosity proved that there was the formation of TFC layer on top of the support PES membrane by IP. The contact angle for support PES membrane was 51.03 ± 3.68° and TFC membrane was 30.08 ± 1.27° as shown in Fig. 4. TFC membrane has smaller contact angle than the PES implying that formation of polyester increases the hydrophilicity of the membrane. The increase in membrane hydrophilicity does favours retention of sugars.16 Past study found that the commercial nanofiltration membranes had PWP between 1.33 and 50.50 L m−2 h−1 bar−1.27 From this study, the membranes prepared had PWP between 0.72 and 33 L m−2 h−1 bar−1 as shown in Table 3, which is within the commercial nanofiltration range as described in the past study.27 The PWP of PES support membrane was 59.54 L m−2 h−1 bar−1, which were not within the nanofiltration PWP. By comparing the PWP between PES support membrane and TFC membrane, there is a notable reduction in PWP of the TFC membrane from the PES support membrane around 44.6% to 98.8%. Again, reduction in PWP was another prove of TFC formation on top of the support membrane.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, temperature at 50 °C, and applied pressure of 5 bar.8b The feed contains 1% wt monosaccharide solution, mass ratio of xylose to glucose in the feed solution at 1
1, temperature at 50 °C, and applied pressure of 5 bar.8b The feed contains 1% wt monosaccharide solution, mass ratio of xylose to glucose in the feed solution at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, temperature of 25 °C, and pressure applied was 4 bar.
1, temperature of 25 °C, and pressure applied was 4 bar.






