Hydrothermal synthesis of a uniform sub-micrometer-spherical Zn0.83Cd0.17S photocatalyst with high activity for photocatalytic hydrogen production†
Abstract
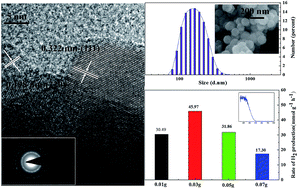
In this work, a series of Zn0.83Cd0.17S with high photocatalytic activity were hydrothermally synthesized and the effects of the hydrothermal temperature on the structural, chemical, morphological properties of the samples were investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), particle size analyzer and X-ray photoelectron spectroscopy (XPS), respectively. The photoabsorption properties were measured using a UV-vis diffused reflectance spectrophotometer and the photocatalytic activities of the samples for hydrogen production were evaluated under 300 W Xe lamp irradiation. The results show that the sub-micro sized spherical Zn0.83Cd0.17S particles are uniform and mainly composed of cubic zinc-blende phase. An increase of temperature improves the crystallinity of the samples and the ratio of Cd and Zn in the solid solution and consequently makes the absorption edges gradually shift monotonically to longer wavelengths. Also, the hydrothermal temperature influences the particle size and distribution of sulfide solid solution. The sample synthesized at 160 °C exhibits the best photocatalytic activity for H2 evolution with a hydrogen production rate of 45.97 mmol h−1 g−1 when the amount of the sample is 0.03 g in a 200 ml aqueous solution containing 0.35 M Na2S and 0.25 M Na2SO3.

 Please wait while we load your content...
Please wait while we load your content...