Thermogravimetric study of sequential carbonation and decarbonation processes over Na2ZrO3 at low temperatures (30–80 °C): relative humidity effect
Abstract
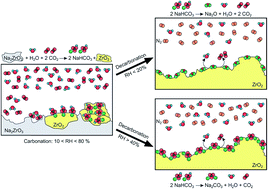
In the present work, Na2ZrO3 was synthetized via a solid-state reaction and characterized by powder X-ray diffraction and N2 physisorption techniques, where desired structural and microstructural characteristics were confirmed. Then, the ceramic material was tested dynamically and isothermally in a low temperature range (30–80 °C) for carbonation and decarbonation processes using relative humidity (RH) values between 0 and 80%. Thermogravimetric results indicate that humidity has a positive influence over the carbonation process, thus the amount of CO2 captured increases as a function of relative humidity. When high values of humidity (70 and 80%) were used, the increase in sample weight was higher than the theoretical amount of 57.2 wt% expected in wet conditions. This result was attributed to the formation of NaHCO3 with a mesoporous microstructure. Then, the relative humidity effect was studied during the decarbonation stage as a sequential step after the carbonation process, using a N2 flow. Infrared spectroscopy and thermogravimetric results showed that NaHCO3 decomposition took place in this process. At low RH values, 0 and 20%, NaHCO3 is decomposed in Na2O and Na2CO3; whereas, when RH was increased between 40 and 80%, only the presence of Na2CO3 was observed. This result indicates that at high humidity conditions the Na2O formation is avoided. Thermal curves show that Na2CO3 decomposition presented a maximum efficiency at 40% of RH, which seems to be the optimal condition for the decarbonation step. Finally, sequential carbonation–decarbonation tests were performed with sodium zirconate samples. Infrared and thermal analyses confirm that it is possible to accomplish successively at least eight cycles of carbonation and decarbonation steps and to obtain high NaHCO3 and Na2CO3 regenerations.

 Please wait while we load your content...
Please wait while we load your content...