An update on hypoallergenicity of peanut and soybean: where are we now?
Muhammad Shamoon†
*a,
Muhammad Wasim Sajidb,
Waseem Safdara,
Junaid Haiderc,
Mukama Omard,
Alfarga Ammara,
Hafiz Rizwan Sharifc,
Saud Khalidf and
Muhammad Atif Randhawa†e
aState Key Laboratory of Food Science and Technology, School of Food Science and Technology, The Synergetic Innovation Center of Food Safety and Nutrition, Jiangnan University, Wuxi 214122, Jiangsu, P. R. China. E-mail: shamoon1096@gmail.com; Tel: +86-132-1879-6692
bDepartment of Biosciences, COMSATS Institute of Information Technology, Sahiwal 57000, Pakistan
cKey Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Food Science and Technology, Jiangnan University, Wuxi 214122, P. R. China
dKey Laboratory of Carbohydrate Chemistry and Biotechnology, School of Biotechnology, Jiangnan University, Wuxi 214122, P. R. China
eFaculty of Food, Nutrition and Home Sciences, National Institute of Food Science & Technology, University of Agriculture, Faisalabad 38040, Pakistan
fCenter for Polymer from Renewable Resources, School of Food Science and Engineering, South China University of Technology, Guangzhou 510640, P. R China
First published on 16th August 2016
Abstract
Legumes are considered as one of the major sources of protein as well as providing other vital components of the diet. At the same time, unfortunately, the leguminous crops peanut and soybean also possess food allergens. The majority of legume allergens originate from four protein families and superfamilies ((i) cupins, (ii) prolamins, (iii) profilins and (iv) pathogenic related proteins larger group) and mainly induce IgE-mediated allergenic reactions. The ace strategy to manage the food allergy strictly encompasses avoiding the food allergens. Apart from numerous other alternative approaches for the treatment of food hypersensitivity, elimination of allergens from food crop(s) is a recent direction of research. Two important approaches have been successfully exploited to develop hypoallergenic leguminous crops: (i) lowering or removing the contents of allergy related proteins via germplasm lines screening and (ii) silencing the allergenic proteins encoding native genes via genetic transformation. Both of these strategies have yielded promising results in the production of peanut and soybean cultivars with low levels of allergic proteins. This current review will elaborate the efforts which have been made to develop hypoallergenic peanut and soybean cultivars to manage the legume allergy with a brief concluding debate on the challenges which still need to be addressed before such products could be launched for consumers in the market place.
Introduction
The Fabaceae (legume) family ranks second in importance only to cereals and grasses and imparts a significant contribution towards staple crop production (∼27%), nutrition, food security and environmental sustainability worldwide.1–3 By virtue, legumes provide both dietary protein and processed vegetable oil as constituents of the human diet. Based on total dry weight, legume seeds are rich in protein content (>20%) in most of the species.4 In addition, they are invaluable sources of industrial oils and livestock feed.5,6 Although the nutritional importance (Table 1) of legumes positively correlates with the development of modern society they pose a serious threat of food allergy among the hypersensitive masses by producing several food allergens and a subsequent economic burden on health care systems around the globe. Among legumes, peanut and soybean allergies are at the apex and the number of scientific publications in recent years reflect the apprehension of researchers towards this issue (Fig. 1). In France, UK, North America and Switzerland peanut allergy is common whereas Japan is stricken with the prevalence of soybean allergy.7,8 In developed world, the current rise in prevalence of food allergy is approaching 10%.9 In USA, this prevalence estimates fall in the range of 1–2% to 10% whereas in Canada the overall rate of food allergy was estimated at 6.7%.10,11 Furthermore, the results from a recent meta analysis of European food allergy prevalence have revealed the overall prevalence of 5.9%.12 The data have also shown that food allergy in children is as high as 10% (developed world) and 7% in rapidly growing Asian societies (China).13 Peanut allergy affects >1% of children and 0.6% of adults, and soybean allergy is problematic to 0.4% of children.14 Additionally, Table 1 displays the other important leguminous crops, besides peanut and soybean, which also possess proteins capable of inducing allergic reactions.| Legume | Bio. name | Allergen(s) | kDa | Protein family | 2S A | 7S G | 11S G | nsLTPs | Nutrition* | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHO (g) | Pro. (g) | Fat (g) | ||||||||||
| a Bio = biological; A = albumins; G = globulins; nsLTPs = nonspecific lipid transfer proteins; *per 100 g dry weight; √ = belongs to specifically indicated family; CHO = carbohydrates; Pro = proteins; PR = pathogenic related; HA = hull allergen; ¶ = these allergens have not been assigned specific biological functions; PSP = plant specific protein; CP = cysteine protease; TPPS = thiol protease of the papain superfamily; SBA = soybean agglutinin; KTI = Kunitz trypsin inhibitor; CS = cupin superfamily (β-conglycinin α-chain of soybean); CS′ = cupin superfamily (β-conglycinin α′-chain of soybean); SBP = seed biotinylated protein; † = 46.5–39.8 (acidic peptide); BP = 25.3–24.3 (basic peptide). | ||||||||||||
| Peanut | Arachis hypogaea | Ara h 1 | 64 | Vicilin | — | √ | — | — | 26.1 | 25.3 | 40.1 | 30–39 |
| Ara h 2 | 17 | Conglutin | √ | — | — | — | ||||||
| Ara h 3 | 60 | Cupin | — | — | √ | — | ||||||
| Ara h 4 | 37 | Cupin | — | — | √ | — | ||||||
| Ara h 5 | 15 | Profilin | — | — | — | — | ||||||
| Ara h 6 | 15 | Conglutin | √ | — | — | — | ||||||
| Ara h 7 | 15 | Conglutin | √ | — | — | — | ||||||
| Ara h 8 | 17 | PR-10 | — | — | — | — | ||||||
| Ara h 9 | 9.8 | nsLTP 1 | — | — | — | √ | ||||||
| Ara h 10 | 16 | Oleosin | — | — | — | — | ||||||
| Ara h agglutinin | 14 | Oleosin | — | — | — | — | ||||||
| Soybean | Glycine max | Gly m 1 | 7 | nsLTP | — | — | — | √ | 20.9 | 43.2 | 19.5 | 30, 33, 36 and 39–49 |
| Gly m 2¶ | 8 | HA | √ | — | — | — | ||||||
| Gly m 3 | 14 | Profilin | — | — | — | — | ||||||
| Gly m 4 | 17 | PR-10 | — | — | — | — | ||||||
| Gly m 5 | 40–70 | Cupin | — | √ | — | — | ||||||
| Gly m 6 | 20–40 | Cupin | — | — | √ | — | ||||||
| Gly m 39¶ | — | PSP | — | — | — | — | ||||||
| Gly m 50¶ | — | HA | — | — | — | — | ||||||
| Gly m Bd 28 K | 28 | Cupin | — | √ | — | — | ||||||
| P34 | 34 | CP | — | — | — | — | ||||||
| Gly m Bd 30 K | 34 | TPPS | — | — | — | — | ||||||
| Gly m lectin | 14.5 | Lectin, SBA | — | — | — | — | ||||||
| Gly m Bd 60 K | 63–67 | Cupin | — | √ | — | — | ||||||
| Gly m glycinin G1 | 40 | Glycinin | — | — | √ | — | ||||||
| Gly m glycinin G2 | 22 | Glycinin | — | — | √ | — | ||||||
| Gly m glycinin G4 | 61.2 | Glycinin | — | — | √ | — | ||||||
| Gly m TI | 20 | KTI | — | — | — | — | ||||||
| Mung bean | Vigna radiata | Vig r 1 | 16 | PR-10 | — | — | — | — | 56.7 | 24 | 1.3 | 39, 50 and 51 |
| Vig 2 | 52 | Cupin | — | — | — | — | ||||||
| Vig r 3 | 50 | Cupin | — | — | — | — | ||||||
| Vig r 4 | 30 | Cupin | — | — | — | — | ||||||
| Vig r 5 | 18 | Profilin | — | — | — | — | ||||||
| Red gram | Cajanus cajan | Caj c 1 | 66 | CS | — | — | — | — | 57.6 | 22.3 | 1.7 | 39 and 51 |
| Caj c 2 | 45 | CS′ | — | — | — | — | ||||||
| Caj c 3 | 45 | CS′ | — | — | — | — | ||||||
| Caj c 4 | 45 | CS′ | — | — | — | — | ||||||
| Caj c 5 | 30 | CS′ | — | — | — | — | ||||||
| Lentil | Lens culinaris | Len c 1 | 48 | Vicilin | — | √ | — | — | 59 | 25.1 | 0.7 | 39, 52 and 53 |
| Len c 2 | 66 | SBP | — | — | — | — | ||||||
| Len c 3 | 9 | nsLTP 1 | — | — | — | — | ||||||
| Lupin | Lupinus angustifolius | Lup an 1 | 55–61 | Conglutin | — | √ | — | — | 9.88 | 15.57 | 2.92 | 39 and 54–56 |
| Lup a vicilin | 66 | Cupin | — | — | — | — | ||||||
| Lup a | 22 | Cupin | — | — | √ | — | ||||||
| Peas | Pisum sativum | Pis s 1 | 44 | Vicilin | — | √ | — | — | 56.5 | 19.7 | 1.1 | 39, 57 and 58 |
| Pis s 2 | 63 | Convicilin | — | √ | — | — | ||||||
| Chickpea | Cicer arietinum | Cis a | 10–12 | Prolamin | √ | — | — | — | 60.9 | 17.1 | 5.3 | 39, 59 and 60 |
| Cis a | † | Cupin | — | — | √ | — | ||||||
Most of the characterized food allergens, proteins or glycoproteins, fall in the range of 5–100 kDa (ref. 15) and to determine their allergenicity yet there is no particular single characteristic has been identified. Structure stability and overall abundance are two oftenly used criterions to predict the allergenic potential.16 The protein which is stable against protease degradation and/or resistant to the heat denaturation is more likely to provoke allergic reaction when goes across the mucosal barriers compared to one which undergoes degradation in gastrointestinal track. Similar is the case for a particular abundant protein (i.e. certain kernel storage proteins; 7S and 11S globulins) and correlation with the speedy elicitation of allergic response.16–18 Nevertheless these characteristics of an allergen contribute to the allergenic capacity; still these criteria are insufficient to predict the allergenicity of a specific protein.16
As food allergy could prove life threatening, in recent years, an approach of taking out allergenic proteins from legume plants has emerged as a unique strategy of its own kind to lessen the hypersensitive responses elicited by unintentional contact with the offending legume allergens. A research based system of developing the genetic stocks with a diminished or “nil” allergenic proteins contents has been intensified during the last decade and thought to be promising in managing the food allergy.19,20 Currently, germplasm lines screening to lower (or to remove) the contents of specific allergy related proteins and silencing the allergenic proteins genes via genetic transformation are two important strategies which could be exploited to achieve the set goals. EcoTILLING (Targeting Induced Local Lesions in Genomes), RNA interference (RNAi) and cosuppression technologies have provided essential platform as experimental and methodological setups to develop hypoallergenic legume cultivars.21–23 Conversely, this approach may be restricted by some factors: (i) queries related to long lasting stability of transgenic suppression of allergenic proteins, (ii) overall consumer acceptability and (iii) regulatory matters associated with genetically modified organisms. Beyond all of these, inducing hypoallergenicity in legumes ultimately proves beneficial in lowering the risks of ever increasing food allergy prevalence. Here in this current review, we have advocated the efforts made on hypoallergenicity of peanut and soybean in order to manage the legume allergy with briefly concluding remarks about the future concerns linked with development of these hypoallergenic legumes.
Legumes allergens classes
From the allergens list available at allergome database24 and extensive previous literature, major allergenic proteins families in legumes and recognized allergenic proteins are listed in Table 1. Concerning with the plant based foods,25 majority of legumes allergens originate from four families and superfamilies of proteins: (i) cupins (included with 7S and 11S globulins), (ii) prolamin superfamily of cereals (composed of 2S storage albumins and nonspecific lipid transfer proteins; nsLTPs), (iii) the profilins (actin binding proteins) and a comparatively larger group of proteins relating to pathogenesis (mainly composed of homologues of the major birch pollen, Bet v 1). The remaining legumes allergenic proteins come from other proteins families i.e. seed biotinylated proteins, cysteine proteases, Kunitz trypsin inhibitors and oleosins etc. (Table 1). Legumes also take the credit of causing some respiratory allergy via cross sensitization with proteins from other species.26,27 Calcium-binding proteins, glucanases (PR-2) and homologues of the Bet v 1 allergen (PR-10) proteins families are most often associated with this phenomenon. Peanut and soybean allergens (Table 1) are mainly considered to trigger the hypersensitivity in food allergy patients, thus majority of the characterized allergens belong to these legumes crops with more severe allergic hypersensitive reactions ascribed with peanut (common cause of anaphylaxis)28 as compared to soybean.29 However, avoiding soy protein is also inevitable because of its frequent use in the commercial food products.Keeping in view their pivotal importance as food allergens sources peanut and soybean have been remained the focus of recent efforts to develop hypoallergenic lines of legumes.
Legumes allergenic response routes
Both IgE and non-IgE pathways can induce the allergenicity in hypersensitive patients. But in case of legumes, the allergenic response is incited by IgE-mediated mechanism and pathogenesis could be sub divided into sensitization and memory induction phase and early/immediate reaction phase (Fig. 2). Recently published data has provided the insights on the pathophysiology and immunological prognosis of food allergy.15,61,62 We have made an effort to schematically present the IgE-mediated allergic responses induced by legumes allergens based on our current understanding of the cellular mechanisms underlying the course of food allergy (Fig. 2). On the other hand, briefly, non IgE facilitated allergic response routes are cell mediated allergenic reactions which are slow in their nature (develop in 6–8 h) and reach to climax at ∼48 h after ingesting the legumes. Non IgE mediated responses mechanisms are mainly eosinophil driven or resulted from T-cells mediated inflammation. Inflammatory mediators are released on T-cells and allergens interaction that provoke the allergy symptoms.63Inducing hypoallergenicity in peanut and soybean
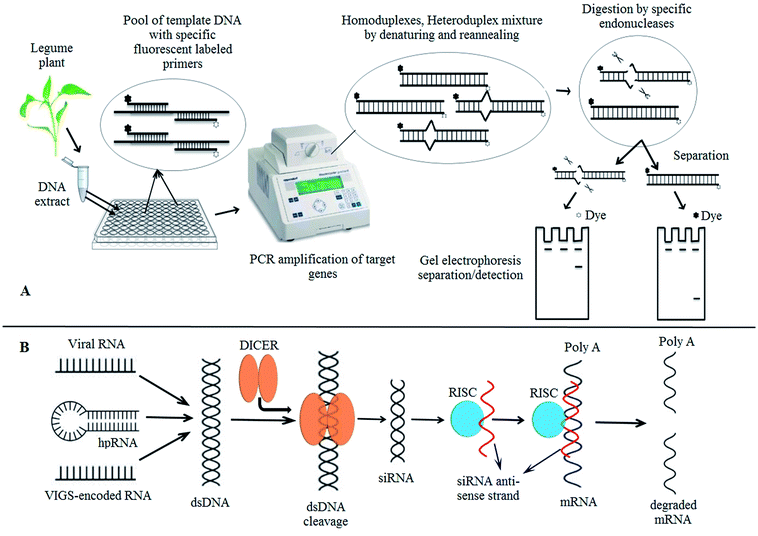
Numerous efforts have been made to develop germplasms with lower allergenic contents. Concerning with peanut and soybean, the focus of the research has been remained in obtaining the varieties with low contents of Ara h 2 (ref. 20, 64 and 65) and P34/Gly m Bd 30 K.66–68 Primarily two approaches have been implemented to produce hypoallergenic legumes: (i) lowering or removing the contents of specific allergy related proteins via germplasm lines screening and (ii) silencing the allergenic proteins encoding native genes via genetic transformation.21–23 Development of germplasm screenings at protein and DNA level has been successfully practiced. To perform the protein screens, highly specific antibodies (polyclonal and/or monoclonal which perceive a particular allergen of interest) or stained gels (which give an estimation of overall protein profile of targeted varieties) are used.69–73 Both of these cases share a common objective to recognize the cultivars with lower or “nil” level of targeted allergen(s). Whereas, at DNA level, the DNA screens have been developed by employing the EcoTILLING strategy (Fig. 3A). EcoTILLING is a molecular technique and variant of TILLING to scrutinize the natural genetic differences in populations of both animals and plants.23,74 This technique depends on amplifying the target gene from pool of differentially labeled template DNAs which may carry natural mutations. Various species are not amenable and this technique aid in discovering the natural variants and putative gene function.23,75 This strategy enables the researchers to quickly screen through many samples with a gene of interest. EcoTILLING has demonstrated a commendable potential to accelerate the identification process of natural alleles of interest that can be used to breed the improved cultivars. Priority objective in developing the DNA screens is to detect the cultivars having natural hypoallergenic variants of already known allergens. Next to EcoTILLING, other approaches such as RNAi technology,65,76 or associated methodologies like cosuppression,77,78 have also been exploited in development of varieties via genetic transformation. RNAi, schematically explained in Fig. 3B, is an endogenous cellular process in which mRNAs are targeted for degradation by dsRNA of identical sequence which leads to silencing of gene79,80 thus interruption in the function of a gene causes diminished protein production (allergens). Primarily employed for knocking down the function of individual targeted genes, this approach has been harnessed in various organisms globally with the production of RNAi libraries to silence most of the genes in their genomes and allowing genome wide loss of function screening. The mechanistic similarities in both of these strategies (RNAi and cosuppression) share common end result of producing abnormal doubled stranded mRNAs which, in turn, elicit the post-transcriptional gene silencing (PTGS). RNAi traditionally uses the anti-sense transgenes to induce PTGS while in cosuppression the induction of PTGS is achieved by a sense transgene. Furthermore, cosuppression is also capable of suppressing the expression of both sense transgene and endogenous homologous genes.81,82 There exist several other differences of mechanistic steps associated with both of these experimental approaches, a comprehensive discussion of these intricate dissimilarities is beyond the scope of this review and readers may consult other sources for further information.79 Leaping from the scope of legumes, many allergens have also been targeted for their reduction in non-legumes crops like apples, tomatoes and rice. They include: Mal d1 (PR-10; apples),83 Lyc e 1, Lyc e 2, Lyc e 3 (profilin, β-fructofuranosidase and nsLTP, respectively; tomatoes),84–86 RAG2, RAP (α-amylase inhibitor; rice)87,88 and several others.89 Concluding with above discussion, it is of worth to notice that transgenic strategies could be potentially implemented to induce hypoallergenicity in peanut and soybean varieties as well as other food crops with an aim to devoid the particular allergenic proteins.Peanut
Peanut (Arachis hypogaea L.) occupies a pivotal position in legumes for providing the food constituents of human diet (Table 1), industrial oils and livestock feed. Hence, the importance of peanut cannot be ignored in healthy development of our present society. Besides countless benefits to its credit, peanut significantly accounts for food allergy. Important allergens from peanut have been listed in Table 1. Food induced anaphylaxis is mainly ascribed with peanut and its frequency seems to be on the rise more. Primarily three allergens of peanut origin i.e. Ara h 1, Ara h 2, Ara h 3 induced allergenic reaction and have been recognized by IgE antibodies in the blood serum of peanut hypersensitive patients.72 Thus, challenge to develop the verities with low levels of these allergens has been mainly addressed in previously conducted researches. Knoll et al.64 have reported lowering of Ara h 1 and Ara h 2 in tetraploid peanut by mutagenesis and TILLING was used to identify the seed traits affecting the mutations. A mutation frequency of 1 SNP/967 kb (single nucleotide polymorphism) was observed using ethyl methanesulfonate as mutagen. The most significant mutations which were observed include disruption of start codon in Ara h 2.02 and a premature stop codon in Ara h 1.02. The absence of Ara h 2.02 was confirmed in succeeding generations and several Ara h 1 isoforms were also removed or reduced as depicted by gel electrophoresis analysis. This work has suggested that TILLING, in peanuts, could be exploited beneficially and extended virtually to any gene i.e. to modify the traits of nutritional properties. Serology of peanut allergy is believed to be different in various parts of the globe. Based on this rationale, thirteen individual plants representing dominant commercial types of peanut (Valencia, Runner, Virginia and Spanish) have been studied to investigate the incidence and abundance of Ara h 1 (range = 12–16%) and Ara h 2 (range = 5.9–93.3%). The results reported non-significant minor differences between the tested samples whereas their binding properties with IgE were similar to great extent. Furthermore, the differences in serology of peanut allergy were independent of difference in allergen composition.73 Conversely, 53 peanut cultivars from China were characterized and their allergenicity was tested in both experimental and clinical trials. The results revealed a broad variation in allergens contents and allergenic potential was affected by allergen composition among the selected cultivars. Less allergenicity was found to be associated with higher Ara h 3/4 (24 kDa), Ara h 2, and lower 3/4 (43, 38, 36 kDa), Ara h 6, respectively. These data provide an insight that screening based on allergen composition may promise to identify the hypoallergenic peanut.90 In another comparatively more extensive study, 60 associations in the U.S.A peanut germplasm collection and 88 from breeding program lines (Florida) were examined to evaluate the levels of three important allergens. None of these selected lines were devoid of Ara h 1, Ara h 2 and Ara h 3 yet some verities possessed fluctuating amounts of these allergens like PI 288210 (lowest Ara h 1 = 70%), PI 372305 (highest Ara h 1 = 18.5%; lowest Ara h 2 = 6.2%) and PI 494795 (highest Ara h 2 = 13.2%; lowest Ara h 3 = 21.8%). These findings suggest that there exist a possible compensation effect in peanut (similar to soybeans) and creating a hypoallergenic peanut lacking of all major allergens is limited with currently available peanut germplasm.72 RNAi technology has been implemented to decrease the quantity of Ara h 2 by transferring the peanut cv. (Georgia Green) with a plasmid construct having portion of the Ara h 2 gene (represented as inverted repeats = 2, 4; Fig. 4B). The Ara h 2 protein was decreased to maximum of 25% from its original amounts leading to ultimate decrease in IgE-binding potential of crude peanut extract as compared to non-transformed plants (control).20 Subsequently, tests were performed on a single generation but no data were obtained to support the stability of transgenic phenotype over multiple generations. The possible explanation could be that gene suppression via RNAi technology involves PTGS and this phenomenon may not always shows stability over generations.81 Hence, further studies are needed to investigate the allergenicity and nutritional profile of subsequent transgenic generations. In another separate study, Chu et al.65 have used the RNAi approach to reduce the levels of two allergens (Ara h 2 and Ara h 6). A vector targeting the both allergens was designed keeping in view the high degree of similarity index between two genes. Microprojectile bombardment was used to carry out the transformation and subsequent gene silencing was evaluated in two generations. The results showed a significant decrease in the level of Ara h 2 and Ara h 6 and their IgE reorganization in sera of three patients. In addition to this, concerning with the seed weight or germination patterns (phenotypic attributes) and protein composition of other allergens (Ara h 1, Ara h 3), no significant dissimilarities was observed between non transgenic and transgenic segregants. Further investigations in some of these transgenic lines showed the decreased level of proteins like conarachin (Ara h 1) and increased amounts of arachin, oleosins, lypoxogenase (Ara h 3).91 In a comparatively larger study, EcoTILLING has been employed for identification of hypoallergenic variants of Ara h 2 in thirty distinct associations of A. duranensis. Gene-specific florescent labeled primers were used to amplify the Ara d 2.01 or Ara h 2.01 from A. duranensis and A. hypogaea genomic DNA pool. Subsequently, amplified products were denatured by heating and randomly reannealed (slow cooling) as a part of TILLING process to stimulate the formation of heteroduplexes and/or homoduplexes if variation exists in nucleotide sequence within the DNA pool. Specific endonucleases digested the mismatched base pairs on double-stranded products and generated fragments were size fractionated and visualized. This investigation resulted in the identification of five different missense mutations in Ara d 2.01. One of them occurred at immunodominant epitope 7 and it was correlated with significant reduction of IgE binding (56–99%).74 Immunotherapy could be suggested as a potential use of this natural hypoallergenic peanut variant because results had confirmed the absence of any mutations occurred within the T-cells epitopes characterized for Ara h 2.01.74 Importantly, development of a commercial peanut variant expressing only hypoallergenic Ara d 2 would be challenging due to the low genetic variability is present in current associations of A. hypogaea (contains 2 isoforms of Ara h 2 other closely related genes like Ara h 6). Thus, it may prove an interesting direction of future research and successful studies in this field may results in achieving a mile stone of producing Ara h 2 devoid (or at least very low level) commercial cultivars of peanut for the betterment of community in perspective of peanut allergy.Soybean
Soybean (Glycine max L.), a miracle food crop with high protein contents, has been used around the globe as nutrition (Table 1), in formulated foods, in pharmaceutical applications and for various other purposes (cooking oil, feed). Apart from its usefulness, soybean also poses a threat of food allergy among the hypersensitive population. Potentially known soybean allergens have been listed in Table 1.Reducing the β-conglycinin and glycinin
A trimeric soybean glycoprotein, β-conglycinin (Gly m 5), is composed of α-(Gly m 5.01), α′-(Gly m 5.02) and β-subunits (Gly m 5.03). It has less methionine level and possesses the undesirable characteristics in preparing the derived products (i.e. tofu), which has accelerated the efforts to decrease β-conglycinin contents in commercial varieties of soybean.92,93 Identifying the α-subunit as an important allergen has also prompted the interest in lowering the β-conglycinin from commercially available soybean cultivars.94 All β-conglycinin and all gycinin subunits deficient soybean variety, QF2 (cross between β-conglycinin deficient natural mutant and glycinin absent inbred line; QY2 × EnB1) has been developed by Takahashi et al.95 QF2 grew, flowered and reproduced normally with no obvious signs of defects despite lacking the two major storage proteins of seed. These findings provided an insight that β-conglycinin and glycinin may not be essential components of the seed. Observation of protein compensation in QF2 had revealed the elevated levels of lipoxygenase, sucrose binding proteins, lectin, P34 and other proteins as compared to ancestor lines. QF2 has also been found to have increased level of free amino acids (particularly arginine), and this excessive production may have been stimulated by the decrease in N2 associated with the loss of β-conglycinin and glycinin. In another separate study, an additional inbred line (EnF2) devoid of all subunits of glycinin as well as α- and α′ subunits of β-conglycinin has been created95 but this line did express a reduce level of β-conglycinin. The reproductive and vegetative developments of EnF2 were normal and its overall amino acids profile was alike to that of QF2. Data representing the possible compensation of other proteins and overall protein composition was not reported in this mutant. As the case with non-transgenic cultivars, transgenic varieties of soybean with low levels of β-conglycinin compensate by synthesis of higher levels of other proteins. This opinion is supported by the fact, when α- and α′ subunits of β-conglycinin elimination is carried out by cosuppression in transgenic soybean the resulting lines accumulate elevated levels of glycinin, proglycinin (precursor form) and P34.96 It is still not well understood that soybean varieties with low β-conglycinin are actually hypoallergenic, given the higher levels of glycinin. Glycine max associations containing elevated contents of both β-conglycinin and glycinin are not compulsory more allergenic when compared to the varieties having normal levels of these proteins. This observation is solely based on IgE antibodies binding profiles without generating the other allergy related data.71 Although majority of the severe soy allergy patients have recognized the status of glycinin as allergen43 yet it remains a question to be answered, whether an increase in glycinin depicts more risk for patients of soy allergy with increased sensitivity or abundance increase may show a quicker sensitization in people (who have not yet developed symptoms) prone to food allergy. Recently, besides following the Genetics based approach, Yang et al.97 have demonstrated the reduction of glycinin and β-conglycinin in soy extract by employing the pulsed ultraviolet light strategy. SDS-PAGE and indirect ELIZA for IgE results suggested a decrease in allergens level in treatment time dependent manner which is probably linked with aggregation. Although pulsed ultraviolet light treatment has provided convincing results yet clinical data is still needed before such non-transgenically produce from soybean cultivars is used in commercial products development.Reducing the P34/Gly m Bd 30 K
The time P34 was recognized as one of the major soybean allergens,98 researchers had started focusing to breed soybean cultivars lacking this protein. Cosuppression technology (Fig. 4A) was used to lessen the P34 protein in soybean cotyledons and P34 was completely removed from transgenic somatic embryos and it was also absent from third generation of homozygous plans. Complete identical patterns of growth, development, reproduction, seed set and seed maturation were observed between transgenic lines and their wild types (controls). Furthermore, P34 deficient transgenic lines protostome exhibited no compensatory protein overexpression. IgE immuneblotting analysis of clinical trial of six soy-allergic patients showed no significant differences in binding of proteins from transgenic and wild type soybeans, though P34 was absent in transgenic lines. Taken together, it may concluded that no additional allergenic proteins were produced as a consequence of genetic modification in removing the P34.78 Generally, it is perceived that genetic engineering may eliminate and/or reduce major food allergens without association of overexpressing the other allergic proteins. In future, the success of this strategy will depend on long term stability of suppression of P34 protein encoding gene expression. The study conducted by Liu et al.99 have successfully demonstrated the feasibility of RNAi in alleviating the soybean allergen. A 395-bp fragment from Gly m bd 30 K gene coding region was designed in binary vector pCAMBIA3301 for pCAMBIA-30 K RNAL plasmid construction. The Gly m Bd 30 K gene was targeted by the selected fragment using RNAi. Soybean cotyledonary node explants were infected with Agrobacterium tumefaciens and transgenic lines were created. qRT-PCR and western blot analysis confirmed the absence of Gly m Bd 30 K, moreover there were no apparent abnormalities were observed in development and phenotype. No doubt transgenic approach has provided encouraging results; the resistance of community to genetically modified products has proved an actual driving force in development of P34 deficient soybean cultivars. Findings of Joseph et al.69 have identified twelve P34 deficient lines from a total of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 266 soybean (G. max), wild annual soybean (Glycine soja Sieb. and Zucc.) and wild perennial Glycine species associations by screening with particular antibody for P34. The lowest levels of P34 were found in PI 603570A and PI 567476 (G. max) and three wild perennial associations. Further characterizing the G. max accessions depicted that P34 levels were less than 1% as compared to standard levels observed in the whole soybean collection.69,71 Other basic seed proteins levels remained unchanged, suggesting absence of compensation effect. P34 cDNA sequence analysis in PI 603570A and PI 567476 has provided a rationale to hypothesize that change in amino acid (serine to cysteine; at 197 position) affects its stability and thereafter abundance in mature seeds. However, recently genomic level investigations has showed that PI 603570A and PI 567476 possess a four base pair nucleotide insertion at start codon gene which is thought to be responsible for low levels of P34, nevertheless the whole molecular mechanism is not fully elucidated.100 Taken all together in conclusion, although it is clear that efforts have been started for incorporating the mutant P34 gene in elite germplasm yet it remains elusive whether such low expression of protein would be tolerated by those with soy hypersensitive. In future, more extensive studies are needed which may prove a land mark of hope in development of commercial hypoallergenic soybean lines and their ultimate utilization in products for consumers.
266 soybean (G. max), wild annual soybean (Glycine soja Sieb. and Zucc.) and wild perennial Glycine species associations by screening with particular antibody for P34. The lowest levels of P34 were found in PI 603570A and PI 567476 (G. max) and three wild perennial associations. Further characterizing the G. max accessions depicted that P34 levels were less than 1% as compared to standard levels observed in the whole soybean collection.69,71 Other basic seed proteins levels remained unchanged, suggesting absence of compensation effect. P34 cDNA sequence analysis in PI 603570A and PI 567476 has provided a rationale to hypothesize that change in amino acid (serine to cysteine; at 197 position) affects its stability and thereafter abundance in mature seeds. However, recently genomic level investigations has showed that PI 603570A and PI 567476 possess a four base pair nucleotide insertion at start codon gene which is thought to be responsible for low levels of P34, nevertheless the whole molecular mechanism is not fully elucidated.100 Taken all together in conclusion, although it is clear that efforts have been started for incorporating the mutant P34 gene in elite germplasm yet it remains elusive whether such low expression of protein would be tolerated by those with soy hypersensitive. In future, more extensive studies are needed which may prove a land mark of hope in development of commercial hypoallergenic soybean lines and their ultimate utilization in products for consumers.
Other legumes
Development of hypoallergenic peanut and soybean (major legumes) lines has been remained main focus of research and still there exists scarcity of efforts reporting to develop other hypoallergenic leguminous crops. Unluckily, there are also no exact estimations of the dominance of other legumes allergies. Mung bean, red gram, lentil, lupin, peas and chickpea also possess some sort of food allergenic proteins (Table 1). Therefore, in perspective to develop their hypoallergenic cultivars, transgenic approaches could equally be taken into account to produce other hypoallergenic legumes.Conclusion and future challenges
Food allergy (more frequent peanut and soybean allergies) is an ever prevailing life threating situation among the hypersensitive people and an economic problem being faced by health care system. Leguminous plants, particularly peanut and soybean yield a broad array of allergic proteins and, generally, IgE antibody binding approach is used to assess the overall allergenicity and/or sensitizing ability caused by altered expression of allergic protein(s). Though some of the characterized allergens are designated as “major allergens”, all of the known allergens must either be reduced or eliminated from a legume variety to be viewed as safe. Various experimental based coping strategies have been practiced to produce hypoallergenic lines of peanut and soybean, and the recently proposed solutions focus mainly on Genetics based ways of eliminating the specific allergens. Allergens deficient peanut and soybean germplasms development and characterization have provided an insight in which transformation could be exploited beneficially in perspective of hypoallergenicity. Transgenic hypoallergenic legumes could reduce the vigilance among hypersensitive people which are stricken with serious food allergy and in future of hypoallergenic legumes; it would be interesting to determine their feasibility for the purpose of end use in products. The future challenges regarding hypoallergenic legumes are diversely vast. Briefly, (i) better understanding on the concept of increased allergenicity due to overabundance of compensatory proteins is inevitable. This is a worth mentioning issue as various compensatory proteins have shown IgE reactive results in same and/or closely related species of plants. (ii) Next apprehension is the likelihood of reversion or reappearance of suppressed trait(s) by silencing of the transgene(s). (iii) Advertisements of products containing ingredients from hypoallergenic legumes should be strictly monitored. Proclaiming that a product is no more allergenic may pose a serious threat for the individuals who are sensitive to proteins different from the removed ones. Furthermore, still there exist scarcity of data reporting the characterization of all potential allergens, and consumers should be educated concerning the production of hypoallergenic legumes and nature of allergens. (iv) The mixture of crop(s) frequently happens in food market worldwide and it is very difficult to certainly partition the legumes between conventional and hypoallergenic sorts. Clear mechanisms should be displayed and followed to avoid misidentification or accidental mixture. One of the possible effective measures could include, for instance, a contract production of hypoallergenic legumes. (v) In westernized lifestyles, factors such as hygiene, lack of exposure to microbial diversity, composition of gut flora, diet, obesity, urban life styles, low vitamin D levels, use of antibiotics and antacids, and environmental chemical exposure also contribute to increasing rate of food allergies.101 Thus, the underlying causes of food allergies are not limited only to legumes allergens but also a generalized Th2-disposition in a susceptible individual. In this context, the development of hypoallergenic legumes may alleviate the burden of food allergies to a small extent. (vi) Finally, assuming the use of hypoallergenic legumes will increase in the future, one cannot rule the emergence of new allergic reactions to either novel or modified allergens from the genetically modified hypoallergenic legumes. Thus, it will be speculating to test the feasibility of produce from hypoallergenic peanut and soybean for its suitability towards therapy purposes in future research. It could be conjectured that a legume deficient in allergen(s) or containing its low levels may not provoke a significant and durable allergenic reaction in hypersensitive patients. Second important point is isomerism (natural mutations) induced hypoallergenicity which affects the production of allergic proteins, and subsequent IgE antibody binding ability, but does not have an obvious effect on overall composition of seed. For the reasons based on above discussed challenges regarding production of hypoallergenic cultivars of legumes, these questions are needed to be addressed in more detail in future studies and we will have a paramount understanding on the scope of benefits associated with hypoallergenicity of legumes after generating more clinical data.Acknowledgements
MS and MAR contributed equally to this work. The authors would like to thanks WS for assistance with proper layout and preparation of the instructive figures. All authors have read and approved the final manuscript. The authors declare no competing/conflict of interest.Notes and references
- S. Daryanto, L. Wang and P.-A. Jacinthe, PLoS One, 2015, 10, e0127401 Search PubMed.
- G. Duc, H. Agrama, S. Bao, J. Berger, V. Bourion, A. M. De Ron, C. L. L. Gowda, A. Mikic, D. Millot, K. B. Singh, A. Tullu, A. Vandenberg, M. C. Vaz Patto, T. D. Warkentin and X. Zong, Crit. Rev. Plant Sci., 2015, 34, 381–411 CrossRef.
- R. K. Varshney, S. M. Mohan, P. M. Gaur, N. Gangarao, M. K. Pandey, A. Bohra, S. L. Sawargaonkar, A. Chitikineni, P. K. Kimurto, P. Janila, K. B. Saxena, A. Fikre, M. Sharma, A. Rathore, A. Pratap, S. Tripathi, S. Datta, S. K. Chaturvedi, N. Mallikarjuna, G. Anuradha, A. Babbar, A. K. Choudhary, M. B. Mhase, C. Bharadwaj, D. M. Mannur, P. N. Harer, B. Z. Guo, X. Q. Liang, N. Nadarajan and C. L. L. Gowda, Biotechnol. Adv., 2013, 31, 1120–1134 CrossRef PubMed.
- M. Duranti, Fitoterapia, 2006, 77, 67–82 CrossRef CAS PubMed.
- P. Phelan, A. P. Moloney, E. J. McGeough, J. Humphreys, J. Bertilsson, E. G. O'Riordan and P. O'Kiely, Crit. Rev. Plant Sci., 2015, 34, 281–326 CrossRef.
- H. Y. Zhu, H. K. Choi, D. R. Cook and R. C. Shoemaker, Plant Physiol., 2005, 137, 1189–1196 CrossRef CAS PubMed.
- S. K. Sathe, C. Q. Liu and V. D. Zaffran, in Annual Review of Food Science and Technology, ed. M. P. Doyle and T. R. Klaenhammer, 2016, vol. 7, pp. 191–220 Search PubMed.
- K. J. Allen and J. J. Koplin, J. Allergy Clin. Immunol., 2016, 4, 215–220 CrossRef PubMed.
- J. Savage and C. B. Johns, Immunol. Allergy Clin. North Am., 2015, 35, 45–59 CrossRef PubMed.
- S. H. Sicherer and H. A. Sampson, J. Allergy Clin. Immunol., 2014, 133, 291–307 CrossRef CAS PubMed.
- L. Soller, M. Ben-Shoshan, D. W. Harrington, J. Fragapane, L. Joseph, Y. St Pierre, S. B. Godefroy, S. La Vieille, S. J. Elliott and A. E. Clarke, J. Allergy Clin. Immunol., 2012, 130, 986–988 CrossRef PubMed.
- B. I. Nwaru, L. Hickstein, S. S. Panesar, A. Muraro, T. Werfel, V. Cardona, A. E. J. Dubois, S. Halken, K. Hoffmann-Sommergruber, L. K. Poulsen, G. Roberts, R. Van Ree, B. J. Vlieg-Boerstra, A. Sheikh and E. F. A. A. Gui, Allergy, 2014, 69, 62–75 CrossRef CAS PubMed.
- S. L. Prescott, R. Pawankar, K. J. Allen, D. E. Campbell, J. K. Sinn, A. Fiocchi, M. Ebisawa, H. A. Sampson, K. Beyer and B.-W. Lee, World Allergy Organ. J., 2013, 6, 21 CrossRef PubMed.
- S. H. Sicherer and H. A. Sampson, J. Allergy Clin. Immunol., 2006, 117, S470–S475 CrossRef CAS PubMed.
- H. Matsuo, T. Yokooji and T. Taogoshi, Allergol. Int., 2015, 64, 332–343 CrossRef CAS PubMed.
- R. E. Goodman, S. Vieths, H. A. Sampson, D. Hill, M. Ebisawa, S. L. Taylor and R. van Ree, Nat. Biotechnol., 2008, 26, 73–81 CrossRef CAS PubMed.
- K. C. M. Verhoeckx, Y. M. Vissers, J. L. Baumert, R. Faludi, M. Feys, S. Flanagan, C. Herouet-Guicheney, T. Holzhauser, R. Shimojo, N. van der Bolt, H. Wichers and I. Kimber, Food Chem. Toxicol., 2015, 80, 223–240 CrossRef CAS PubMed.
- H. Breiteneder and E. N. C. Mills, J. Allergy Clin. Immunol., 2005, 115, 14–23 CrossRef CAS PubMed.
- M. B. Singh and P. L. Bhalla, Trends Plant Sci., 2008, 13, 257–260 CrossRef CAS PubMed.
- H. W. Dodo, K. N. Konan, F. C. Chen, M. Egnin and O. M. Viquez, Plant Biotechnol. J., 2008, 6, 135–145 CrossRef CAS PubMed.
- M. Boutros and J. Ahringer, Nat. Rev. Genet., 2008, 9, 554–566 CrossRef CAS PubMed.
- B. L. Davidson and P. B. McCray Jr, Nat. Rev. Genet., 2011, 12, 329–340 CrossRef CAS PubMed.
- N. A. Barkley and M. L. Wang, Curr. Genomics, 2008, 9, 212–226 CrossRef CAS PubMed.
- A. Mari and D. Riccioli, J. Allergy Clin. Immunol., 2004, 113, S301 Search PubMed.
- A. Harrer, M. Egger, G. Gadermaier, A. Erler, M. Hauser, F. Ferreira and M. Himly, Mol. Nutr. Food Res., 2010, 54, 93–112 CAS.
- A. M. Kazatsky and R. A. Wood, Curr. Allergy Asthma Rep., 2016, 16, 22 CrossRef PubMed.
- R. Uotila, A. K. Kukkonen, A. S. Pelkonen and M. J. Makela, Allergy, 2016, 71, 514–521 CrossRef CAS PubMed.
- S. Quirce and A. Fiandor, Curr. Opin. Allergy Clin. Immunol., 2016, 16, 86–92 CrossRef CAS PubMed.
- M. Sato, A. Shukuya, S. Sato, T. Komata, T. Utsunomiya, T. Imai, M. Tomikawa and M. Ebisawa, Allergol. Int., 2016, 65, 68–73 CrossRef PubMed.
- A. K. Verma, S. Kumar, M. Das and P. D. Dwivedi, Clin. Rev. Allergy Immunol., 2013, 45, 30–46 CrossRef CAS PubMed.
- A. W. Burks, L. W. Williams, R. M. Helm, C. Connaughton, G. Cockrell and T. Obrien, J. Allergy Clin. Immunol., 1991, 88, 172–179 CrossRef CAS PubMed.
- T. Kleber-Janke, R. Crameri, U. Appenzeller, M. Schlaak and W. M. Becker, Int. Arch. Allergy Appl. Immunol., 1999, 119, 265–274 CrossRef CAS.
- T. Jin, F. Guo, Y.-W. Chen, A. Howard and Y.-Z. Zhang, Mol. Immunol., 2009, 46, 1796–1804 CrossRef CAS PubMed.
- T. Kleber-Janke, R. Crameri, S. Scheurer, S. Vieths and W. M. Becker, J. Chromatogr. B: Biomed. Sci. Appl., 2001, 756, 295–305 CrossRef CAS.
- F. van Wijk, S. Hartgring, S. J. Koppelman, R. Pieters and L. M. J. Knippels, Clin. Exp. Allergy, 2004, 34, 1422–1428 CrossRef CAS PubMed.
- D. Mittag, J. Akkerdaas, B. K. Ballmer-Weber, L. Vogel, M. Wenising, W. M. Becker, S. J. Koppelman, A. C. Knulst, A. Helbling, S. L. Hefle, R. van Ree and S. Vieths, J. Allergy Clin. Immunol., 2004, 114, 1410–1417 CrossRef CAS PubMed.
- R. Asero, G. Mistrello, D. Roncarolo, S. Amato, G. Caldironi, F. Barocci and R. van Ree, Allergy, 2002, 57, 900–906 CrossRef CAS PubMed.
- L. Pons, C. Chery, A. Romano, F. Namour, M. C. Artesani and J. L. Gueant, Allergy, 2002, 57, 88–93 CrossRef PubMed.
- C. Gopalan, B. V. R. Sastri and S. C. Balasubramanian, Nutritive Value of Indian Foods, National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, 1989 Search PubMed.
- R. M. Helm, G. Cockrell, E. Herman, A. W. Burks, H. A. Sampson and G. A. Bannon, Int. Arch. Allergy Appl. Immunol., 1998, 117, 29–37 CrossRef CAS.
- R. Codina, R. F. Lockey, E. FernandezCaldas and R. Rama, Clin. Exp. Allergy, 1997, 27, 424–430 CrossRef CAS PubMed.
- A. Ogawa, M. Samoto and K. Takahashi, J. Nutr. Sci. Vitaminol., 2000, 46, 271–279 CrossRef CAS PubMed.
- T. Holzhauser, O. Wackermann, B. K. Ballmer-Weber, C. Bindslev-Jensen, J. Scibilia, L. Perono-Garoffo, S. Utsumi, L. K. Poulsen and S. Vieths, J. Allergy Clin. Immunol., 2009, 123, 452–458 CrossRef CAS PubMed.
- K. Ito, S. Sjölander, S. Sato, R. Movérare, A. Tanaka, L. Söderström, M. Borres, M. Poorafshar and M. Ebisawa, J. Allergy Clin. Immunol., 2011, 128, 673–675 CrossRef CAS PubMed.
- D. Mittag, V. Batori, P. Neudecker, R. Wiche, E. P. Friis, B. K. Ballmer-Weber, S. Vieths and E. L. Roggen, Mol. Immunol., 2006, 43, 268–278 CrossRef CAS PubMed.
- H. Tsuji, N. Bando, M. Hiemori, R. Yamanishi, M. Kimoto, K. Nishikawa and T. Ogawa, Biosci., Biotechnol., Biochem., 1997, 61, 942–947 CrossRef CAS PubMed.
- H. Tsuji, N. Okada, R. Yamanishi, N. Bando, M. Kimoto and T. Ogawa, Biosci., Biotechnol., Biochem., 1995, 59, 150–151 CrossRef CAS PubMed.
- R. Codina, R. F. Lockey, E. Fernandez-Caldas and R. Rama, Int. Arch. Allergy Appl. Immunol., 1999, 119, 69–71 CrossRef CAS.
- S. S. Natarajan, C. P. Xu, H. H. Bae, T. J. Caperna and W. A. Garrett, J. Agric. Food Chem., 2006, 54, 3114–3120 CrossRef CAS PubMed.
- D. Mittag, S. Vieths, L. Vogel, D. Wagner-Loew, A. Starke, P. Hunziker, W. M. Becker and B. K. Ballmer-Weber, Clin. Exp. Allergy, 2005, 35, 1049–1055 CrossRef CAS PubMed.
- A. Misra, R. Kumar, V. Mishra, B. P. Chaudhari, S. Raisuddin, M. Das and P. D. Dwivedi, Clin. Exp. Allergy, 2011, 41, 1157–1168 CrossRef CAS PubMed.
- R. Sanchez-Monge, C. Y. Pascual, A. Diaz-Perales, J. Fernandez-Crespo, M. Martin-Esteban and G. Salcedo, J. Allergy Clin. Immunol., 2000, 106, 955–961 CrossRef CAS PubMed.
- E. I. Finkina, S. V. Balandin, M. V. Serebryakova, N. A. Potapenko, A. A. Tagaev and T. V. Ovchinnikova, Biochemistry, 2007, 72, 430–438 CAS.
- K. A. B. M. Peeters, S. J. Koppelman, E. van Hoffen, C. W. H. van der Tas, C. F. den Hartog Jager, A. H. Penninks, S. L. Hefle, C. A. F. M. Bruijnzeel-Koomen, E. F. Knol and A. C. Knulst, Clin. Exp. Allergy, 2007, 37, 108–115 CrossRef CAS PubMed.
- D. E. Goggin, G. Mir, W. B. Smith, M. Stuckey and P. M. C. Smith, J. Agric. Food Chem., 2008, 56, 6370–6377 CrossRef CAS PubMed.
- C. Magni, A. Herndl, E. Sironi, A. Scarafoni, C. Ballabio, P. Restani, R. Bernardini, E. Novembre, A. Vierucci and M. Duranti, J. Agric. Food Chem., 2005, 53, 4567–4571 CrossRef CAS PubMed.
- R. Vanree, V. Voitenko, W. A. Vanleeuwen and R. C. Aalberse, Int. Arch. Allergy Appl. Immunol., 1992, 98, 97–104 CrossRef CAS.
- R. Sanchez-Monge, G. Lopez-Torrejon, C. Y. Pascual, J. Varela, M. Martin-Esteban and G. Salcedo, Clin. Exp. Allergy, 2004, 34, 1747–1753 CrossRef CAS PubMed.
- J. Vioque, R. Sanchez-Vioque, A. Clemente, J. Pedroche, J. Bautista and F. Millan, J. Agric. Food Chem., 1999, 47, 1405–1409 CrossRef CAS PubMed.
- F. Karamloo, A. Wangorsch, H. Kasahara, L. B. Davin, D. Haustein, N. G. Lewis and S. Vieths, Eur. J. Biochem., 2001, 268, 5310–5320 CrossRef CAS PubMed.
- B. J. Pelz and P. J. Bryce, Pediatr. Clin. North Am., 2015, 62, 1363–1375 CrossRef PubMed.
- L. K. Johnston, K. B. Chien and P. J. Bryce, J. Immunol., 2014, 192, 2529–2534 CrossRef CAS PubMed.
- K. Preece, A. Blincoe, E. Grangaard, G. T. Ostring, D. Purvis, A. Shiek, J. Sinclair and R. Winkler, N. Z. Med. J., 2016, 129, 78–88 Search PubMed.
- J. E. Knoll, M. L. Ramos, Y. Zeng, C. C. Holbrook, M. Chow, S. Chen, S. Maleki, A. Bhattacharya and P. Ozias-Akins, BMC Plant Biol., 2011, 11, 81 CrossRef CAS PubMed.
- Y. Chu, P. Faustinelli, M. Laura Ramos, M. Hajduch, S. Stevenson, J. J. Thelen, S. J. Maleki, H. Cheng and P. Ozias-Akins, J. Agric. Food Chem., 2008, 56, 11225–11233 CrossRef CAS PubMed.
- S. Liu, G. Chen, L. Yang, J. Gai and Y. Zhu, J. Pure Appl. Microbiol., 2013, 7, 589–599 CAS.
- K.-H. Jeong, M.-S. Choi, S.-K. Lee, M.-J. Seo, T.-Y. Hwang, H.-T. Yun, H.-S. Kim, J.-T. Kim, Y.-U. Kwon and Y.-H. Kim, Plant Breed. Biotechnol., 2013, 1, 298–306 CrossRef.
- Y.-M. Wu, R.-X. Guan, Z.-X. Liu, R.-Z. Li, R.-Z. Chang and L.-J. Qiu, J. Integr. Plant Biol., 2012, 54, 4–14 CrossRef CAS PubMed.
- L. M. Joseph, T. Hymowitz, M. A. Schmidt and E. M. Herman, Crop Sci., 2006, 46, 1755–1763 CrossRef CAS.
- A. Clemente, M. C. Arques, M. Dalmais, C. Le Signor, C. Chinoy, R. Olias, T. Rayner, P. G. Isaac, D. M. Lawson, A. Bendahmane and C. Domoney, PLoS One, 2015, 10, e0138039 Search PubMed.
- R. W. Yaklich, R. M. Helm, G. Cockrell and E. M. Herman, Crop Sci., 1999, 39, 1444–1447 CrossRef CAS.
- I.-H. Kang, M. Gallo and B. L. Tillman, Crop Sci., 2007, 47, 997–1003 CrossRef CAS.
- S. J. Koppelman, R. A. A. Vlooswijk, L. M. J. Knippels, M. Hessing, E. F. Knol, F. C. van Reijsen and C. Bruijnzeel-Koomen, Allergy, 2001, 56, 132–137 CrossRef CAS PubMed.
- M. L. Ramos, J. J. Huntley, S. J. Maleki and P. Ozias-Akins, Plant Mol. Biol., 2009, 69, 325–335 CrossRef CAS PubMed.
- L. Comai, K. Young, B. J. Till, S. H. Reynolds, E. A. Greene, C. A. Codomo, L. C. Enns, J. E. Johnson, C. Burtner, A. R. Odden and S. Henikoff, Plant J., 2004, 37, 778–786 CrossRef CAS PubMed.
- J. H. Sherman, T. Munyikwa, S. Y. Chan, J. S. Petrick, K. W. Witwer and S. Choudhuri, Regul. Toxicol. Pharmacol., 2015, 73, 671–680 CrossRef PubMed.
- O. Yu, J. Shi, A. O. Hession, C. A. Maxwell, B. McGonigle and J. T. Odell, Phytochemistry, 2003, 63, 753–763 CrossRef CAS PubMed.
- E. M. Herman, R. M. Helm, R. Jung and A. J. Kinney, Plant Physiol., 2003, 132, 36–43 CrossRef CAS PubMed.
- A. Fire, S. Q. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver and C. C. Mello, Nature, 1998, 391, 806–811 CrossRef CAS PubMed.
- E. J. Chapman and J. C. Carrington, Nat. Rev. Genet., 2007, 8, 884–896 CrossRef CAS PubMed.
- G. J. Hannon, Nature, 2002, 418, 244–251 CrossRef CAS PubMed.
- M. Kusaba, Curr. Opin. Biotechnol., 2004, 15, 139–143 CrossRef CAS PubMed.
- A. E. J. Dubois, G. Pagliarani, R. M. Brouwer, B. J. Kollen, L. O. Dragsted, F. D. Eriksen, O. Callesen, L. J. W. J. Gilissen, F. A. Krens, R. G. F. Visser, M. J. M. Smulders, B. J. Vlieg-Boerstra, B. J. Flokstra-de Blok and W. E. van de Weg, Allergy, 2015, 70, 1406–1412 CrossRef CAS PubMed.
- H. Kaulfuerst-Soboll, M. Mertens, R. Brehler and A. von Schaewen, PLoS One, 2011, 6, e17800 CAS.
- V. Mahler, L. Q. Le, Y. Lorenz, S. Scheurer, S. Biemelt, S. Vieths and U. Sonnewald, Exp. Dermatol., 2007, 16, 199 Search PubMed.
- L. Q. Le, Y. Lorenz, S. Scheurer, K. Fotisch, E. Enrique, J. Bartra, S. Biemelt, S. Vieths and U. Sonnewald, Plant Biotechnol. J., 2006, 4, 231–242 CrossRef CAS PubMed.
- S. Kurokawa, M. Kuroda, M. Mejima, R. Nakamura, Y. Takahashi, H. Sagara, N. Takeyama, S. Satoh, H. Kiyono, R. Teshima, T. Masumura and Y. Yuki, Plant Cell Rep., 2014, 33, 75–87 CrossRef CAS PubMed.
- Y. Tada, H. Akagi, T. Fujimura and T. Matsuda, Breed. Sci., 2003, 53, 61–67 CrossRef CAS.
- A. K. G. Hassan and Y. P. Venkatesh, European Annals of Allergy and Clinical Immunology, 2015, 47, 180–187 CAS.
- Z. Wu, N. Zhou, F. Xiong, X. Li, A. Yang, P. Tong, R. Tang and H. Chen, Food Chem., 2016, 196, 459–465 CrossRef CAS PubMed.
- S. E. Stevenson, Y. Chu, P. Ozias-Akins and J. J. Thelen, J. Proteomics, 2009, 72, 555–566 CrossRef CAS PubMed.
- Y.-H. Hsiao, C.-P. Lu, M.-I. Kuo and J.-F. Hsieh, Food Chem., 2016, 200, 55–61 CrossRef CAS PubMed.
- W.-S. Kim, J. M. Jez and H. B. Krishnan, Frontiers in Plant Science, 2014, 5, 633 CrossRef PubMed.
- A. L. Capriotti, G. Caruso, C. Cavaliere, R. Samperi, S. Stampachiacchiere, R. Z. Chiozzi and A. Lagana, J. Agric. Food Chem., 2014, 62, 9893–9899 CrossRef CAS PubMed.
- M. Takahashi, Y. Uematsu, K. Kashiwaba, K. Yagasaki, M. Hajika, R. Matsunaga, K. Komatsu and M. Ishimoto, Planta, 2003, 217, 577–586 CrossRef CAS PubMed.
- A. J. Kinney, R. Jung and E. M. Herman, Plant Cell, 2001, 13, 1165–1178 CAS.
- W. W. Yang, S. Y. Chung, O. Ajayi, K. Krishnamurthy, K. Konan and R. Goodrich-Schneider, Int. J. Food Eng., 2010, 6, 11 Search PubMed.
- T. Ogawa, N. Bando, H. Tsuji, H. Okajima, K. Nishikawa and K. Sasaoka, J. Nutr. Sci. Vitaminol., 1991, 37, 555–565 CrossRef CAS PubMed.
- S. C. Liu, G. H. Chen, L. F. Yang, J. Y. Gai and Y. L. Zhu, J. Pure Appl. Microbiol., 2013, 7, 589–599 CAS.
- K. Bilyeu, C. Ren, H. T. Nguyen, E. Herman and D. A. Sleper, Plant Genome, 2009, 2, 141–148 CrossRef CAS.
- S. Benede, A. B. Blazquez, D. Chiang, L. Tordesillas and M. C. Berin, EBioMedicine, 2016, 7, 27–34 CrossRef PubMed.
Footnote |
| † These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |