Interaction between bile salt sodium glycodeoxycholate and PEO–PPO–PEO triblock copolymers in aqueous solution†
S. Bayatia,
C. Anderberg Haglund‡
a,
N. V. Pavelb,
L. Galantinib and
K. Schillén*a
aDivision of Physical Chemistry, Department of Chemistry, Lund University, P.O. Box 124, SE-221 00 Lund, Sweden. E-mail: Karin.Schillen@fkem1.lu.se
bDepartment of Chemistry, “Sapienza” University of Rome, P. le A. Moro 5, 00185 Rome, Italy
First published on 6th July 2016
Abstract
The interactions of the anionic bile salt NaGDC with three triblock copolymers based on poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO), denoted P65, P123 and F127, were investigated using high-sensitive differential scanning calorimetry (DSC), turbidimetry, dynamic and static light scattering and small angle X-ray scattering (SAXS). P65 and P123 had the same hydrophilic PEO block lengths, whereas F127 and P123 had the same hydrophobic PPO block length. In water, the block copolymers self-assembled and formed spherical micelles at a critical micelle temperature, which depended on both the PPO/PEO composition ratio and the molecular weight of the copolymer. The mixed systems were studied at a constant P65, P123 or F127 concentration (i.e., 1.0 wt% or 5.0 wt%) with varying nNaGDC/npolymer molar ratio (MR) from 0 to 12. The DSC measurements presented endothermic enthalpy values (correlated to the amount of PPO that dehydrates in the aggregation process) that were suppressed at high MR. At 50 °C, the NaGDC molecules associated to the PPO core – PEO corona interface of the copolymer micelle forming a negatively charged block copolymer micelle–NaGDC complex. The complexes began to disintegrate upon NaGDC addition. Their resistance to disruption followed the stability order as inferred from the CMT values. At 20 °C, the unassociated block copolymer chains interacted with the NaGDC micelles and formed small NaGDC-rich complexes with a radius of ∼2 nm as determined by SAXS.
Introduction
The key property of matter in order to get a hierarchical architecture is self-assembly. In macromolecular soft matter systems, self-assembly gives rise to unique structures and functionality that are important for both biological and technological applications. A specific family of self-assembling macromolecules is block copolymers.1 They can self-organise into chemically different domains either in the bulk or in a solvent that is selective for one kind of the macromolecule's blocks. The size and periodicity of these domains are within the nanometre scale (about 10 nm and below), and the final nanostructure depends on the properties of each block, i.e., their chain length and volume fraction, and their mutual compatibility as well as the solvent.2 Because of this, self-assembling macromolecules are of interest for both fundamental studies and for the development of nanotechnologies with promising benefits in civil society, e.g., mesoporous materials3 or nanostructured materials for chemosensing to mention a few. Non-toxic and stimuli-responsive block copolymers, that withstand human interaction and thus can be used to avoid potential health risks, have applications in the biomedical field, as drug delivery systems, as biological response modifiers in chemotherapy or as agents for non-viral gene therapy. In these applications poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymers (EOn–POm–EOn) have proven particularly successful.4,5 Belonging to the family of nonionic surfactants, the self-assembly phase behavior in water of PEO–PPO–PEO block copolymers is highly dependent on temperature.6 This fact is based on the conformational changes of the EO (and PO) unit; with increased probability of less polar conformations at higher temperatures making the interaction of PEO with water less favorable.7–10 It is now well-established that they form different structures with a dehydrated PPO domain, i.e., micelles or vesicles, and a variety of liquid crystalline structures depending on the concentration, see for instance ref. 11–20 and the references therein.Specific synergy effects can be obtained when environmental-friendly block copolymers are mixed with surfactants. Such mixtures have a very wide application area and are found in many household and industrial products. Depending on which type of feature that is desired, their synergistic properties can be utilised in cosmetics, food products and detergents. Two examples of particularly interesting mixtures are those containing PEO–PPO–PEO block copolymers. These form mixed core–shell micelles with nonionic surfactants of the CiEOj type that change morphology depending on the surfactant content, which in turn can be used to control the rheological performance of industrial products.21 The association behaviour and the induced break-up of the PEO–PPO–PEO micelles when mixed with ionic surfactants have attracted great interest in literature.22–41 In order to precisely control the mechanism of self-organisation, it is crucial to gain understanding of the thermodynamics of the self-assembly in these polymer systems and to characterise of the nanostructures formed both in solution and at interfaces. One also needs to determine the intermolecular interactions at play.
One of the applications of PEO–PPO–PEO block copolymers is their use in drug formulations. When introduced in living organisms they interact with many different biological molecules. These interactions are bound to affect the self-assembly of the copolymers and therefore their efficacy as drug carriers. The interaction with biological surfactants such as bile salts is particular significant, as the remarkable effects of classical surfactants on the self-assembly of block copolymers are known since previously. Bile salts are anionic steroid surfactants, naturally present in the human body. These are synthesised in the human liver, stored in the gall-bladder and introduced in the small intestine to help the body digest fat while undergoing a recycling circulation.42,43 As a result of their unusual amphiphilic structure, bile salts self-assemble to form micelles, gels, and fibres with increased concentration in aqueous solution.44,45 An additional interest for studying PEO–PPO–PEO–bile salt interactions is related to the potential application of these block copolymers as bile salt sequestrants in the treatment of bile acid diarrhoea and hypercholesterolemia, and where the bile salt concentration level needs to be controlled.46,47 Block copolymer micelles could in fact be used as carriers of bile salts to transport them out of the body as opposed to the currently used sequestrants based on cationic polymers.48,49 Finally, it is worth to remark that block copolymer–bile salt mixed aggregates could constitute themselves biocompatible drug carriers, which further motivate the investigation of PEO–PPO–PEO–bile salt mixtures.
The purpose of the present study was to investigate the effect of the bile salt sodium glycodeoxycholate (NaGDC) on the self-assembly behavior and its dependence on block length and molecular weight of the block copolymers P65 (EO19PO29EO19), F127 (EO97PO69EO97) and P123 (EO20PO68EO20) using differential scanning calorimetry (DSC) and dynamic light scattering (DLS). We also performed a detailed small angle X-ray scattering (SAXS) investigation of the structure of the different mixed complexes that were formed. Similarly to conventional surfactants, it has been recently shown that bile salts interact with PEO–PPO–PEO block copolymers to form mixed complexes in dilute aqueous solutions that in turn disintegrate at high concentrations of bile salt.50–52 This work is expected to clarify the effect of the block copolymer structure on the stability of these complexes.
Experimental
Materials and sample preparation
The poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymers P65 (EO19PO29EO19), P123 (EO20PO68EO20) and F127 (EO97PO69EO97), with the nominal molar mass of 3400, 5750 and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1 respectively, were donated by BASF Corporation Performance Chemicals (Mount Olive, New Jersey, US). The bile salt, sodium glycodeoxycholate, NaGDC (M = 471.61 g mol−1, assay ≥97%) was purchased from Sigma-Aldrich. The molecular structures of the investigated substances are displayed in Scheme 1. No further purification was performed on the materials. Water, purified with a Milli-Q system (Millipore Corporation, Bedford, MA) equipped with a 0.22 μm filter, was used as a solvent in all solutions. The stock solutions of the block copolymers and NaGDC were prepared by weighing. They were equilibrated in a cold room at 5–6 °C and at room temperature respectively for at least 4 h before preparation of the mixed solutions, which were thereafter equilibrated in the cold room for at least 12 h. In the case of mixtures, the block copolymer concentration was fixed to either 1.00 wt% (corresponding to 2.94, 1.74 and 0.792 mM for P65, P123 and F127, respectively) or 5.00 wt% (i.e., 14.7, 8.68 and 3.96 mM for P65, P123 and F127 respectively) while the concentration of NaGDC varied between 0 and 177 mM, 0–104 mM and 0–47.6 mM for P65, P123 and F127 respectively. The former were prepared by dilution of the 5.0 wt% solutions. The molar ratios (MR = nNaGDC/npolymer) of 0, 0.3, 0.6, 3 and 12 were kept the same for all block copolymer mixtures. The solutions of P65 in water were prepared with the following concentrations: 0.50, 1.00, 2.50, 5.00, and 10.0 wt% (i.e., 1.47, 2.94, 7.35, 14.7 and 29.4 mM, respectively).
600 g mol−1 respectively, were donated by BASF Corporation Performance Chemicals (Mount Olive, New Jersey, US). The bile salt, sodium glycodeoxycholate, NaGDC (M = 471.61 g mol−1, assay ≥97%) was purchased from Sigma-Aldrich. The molecular structures of the investigated substances are displayed in Scheme 1. No further purification was performed on the materials. Water, purified with a Milli-Q system (Millipore Corporation, Bedford, MA) equipped with a 0.22 μm filter, was used as a solvent in all solutions. The stock solutions of the block copolymers and NaGDC were prepared by weighing. They were equilibrated in a cold room at 5–6 °C and at room temperature respectively for at least 4 h before preparation of the mixed solutions, which were thereafter equilibrated in the cold room for at least 12 h. In the case of mixtures, the block copolymer concentration was fixed to either 1.00 wt% (corresponding to 2.94, 1.74 and 0.792 mM for P65, P123 and F127, respectively) or 5.00 wt% (i.e., 14.7, 8.68 and 3.96 mM for P65, P123 and F127 respectively) while the concentration of NaGDC varied between 0 and 177 mM, 0–104 mM and 0–47.6 mM for P65, P123 and F127 respectively. The former were prepared by dilution of the 5.0 wt% solutions. The molar ratios (MR = nNaGDC/npolymer) of 0, 0.3, 0.6, 3 and 12 were kept the same for all block copolymer mixtures. The solutions of P65 in water were prepared with the following concentrations: 0.50, 1.00, 2.50, 5.00, and 10.0 wt% (i.e., 1.47, 2.94, 7.35, 14.7 and 29.4 mM, respectively).
 | ||
| Scheme 1 Molecular structures of sodium glycodeoxycholate (NaGDC) and poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymer (EOn–POm–EOn). | ||
Differential scanning calorimetry
DSC measurements were performed using a high sensitivity MicroCal™ VP-DSC (Microcal, Northampton, MA).50,53 See the ESI† for the Experimental conditions.Dynamic and static light scattering
An ALV/DLS/SLS-5022F, CGF-8F-based compact goniometer (ALV-GmbH, Langen, Germany) was employed to perform the dynamic and static light scattering measurements. The complete information about the instrument and experimental conditions can be found in ref. 50 and 54. Details of the DLS data analysis are given in ESI.†Small angle X-ray scattering
Small angle X-ray scattering measurements were carried out at MAX II SAXS beamline I911-4 at the MAXIV Laboratory in Lund, Sweden. The complete information about the instrument and the experimental settings can be found in ref. 50 and 55. To obtain a better signal-to-noise ratio, all measurements were performed on mixtures containing 5.0 wt% of block copolymer and with molar ratios ranging from 0 to 12. In order to understand the behaviour of the mixtures in connection with the DSC results, experiments were performed at two temperatures of 20 ± 0.5 °C and 50 ± 0.5 °C. The scattering intensity was corrected for the background (intensity contribution of the solvent and the empty capillary) and put in the absolute scale considering water as a calibration standard. The strategy of the data analysis is described in ESI.†Results and discussion
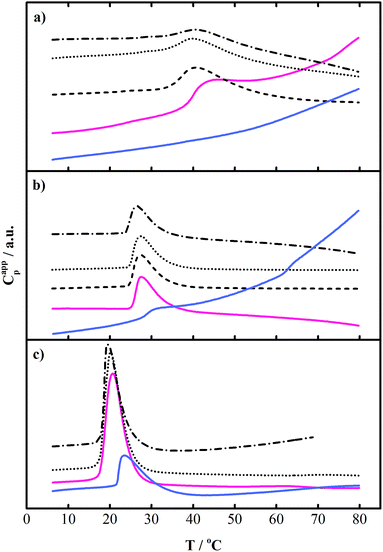
The self-assembly of the PEO–PPO–PEO copolymers P123 and F127 in water has been extensively studied and we refer to for instance ref. 13, 23, 28, 31 and 56–62 for further information. In short, they both form micelles with a PPO core and a PEO corona above a critical micelle concentration (CMC) and a critical micelle temperature (CMT). The latter is easily detected in a DSC experiment due to the endothermic signal, which is mainly related to the dehydration of the PPO blocks.15,57,63 The micellization process is driven both by a decrease in polarity of the PEO and PPO blocks due to their conformational changes as the temperature increases8 and by the large entropy gain from the release of water in the process. From DLS measurements, the hydrodynamic radius of the P123 and F127 micelles has been determined to be about 9–12 nm depending on the temperature and on the polymer batch.21,28,50,51,56,57,64–66The aqueous solution properties of P65 have been less explored.13,60,67–69 Therefore, before starting our investigation of the mixed systems, we performed a minor study of the self-assembly behavior of our P65 batch combining DSC and DLS (see below). Furthermore, to inspect our F127 batch and compare the results to the literature, DSC measurements on two concentrations of F127 (i.e., 1.0 wt% and 5.0 wt%) were carried out in the same temperature interval as for P65. In the case of P123, the same sample batch as in ref. 50 and 51 was used and we refer to that work for more information about the micelle formation of this block copolymer.
Self-association of P65 copolymer in water
The values of the enthalpy of micellization ΔHtr (from the peak area), CMT (≡ the onset temperature, Tonset) and Tm (the peak maximum) are listed in Table S1, ESI.† The width of the peak is related to the cooperativity of the process, how many unimers that are involved, the copolymer polydispersity, as well as the change in molecular weight of the micelles in the corresponding temperature interval, see, e.g., ref. 50 and the references therein. The small peak before the main transition that was observed for all concentrations has been proposed to be associated with the thermally induced break-up of large aggregates of lower-size contaminants of copolymer that exist at temperatures below the Tonset of micelle formation70 (see also the DLS results below). The scattered data at the end of the temperature interval is due to the difficulties in setting the base line in addition to the approach of the phase separation temperature (i.e., the cloud point), which is around 82 °C according to the manufacturer.71
As noticed, Tonset and Tm decreased with increasing block copolymer concentration, while ΔHtr increased as more P65 unimers became involved in the micellization process. This trend has been observed in multiple studies before. For evaluated calorimetric data of 1.0 wt% and 5.0 wt% F127 in water, see Tables S4 and S5, ESI.† The data for both polymers were all in the same range as the previously reported values, see e.g. ref. 13, 31, 58, 60, 61 and 72. They varied to a minor extent due to the different batches used (and due to how the CMT was extracted from the experiment). In this work, we obtained ΔHtr = 81 kJ mol−1 and 310 kJ mol−1 for 1.0 wt% solutions of P65 and F127, respectively (Tables S1 and S4, ESI†). These values can be compared to 426 kJ mol−1 previously obtained for P123 using the same instrument.50 The CMT of a 1.0 wt% P65 solution was 38.1 °C, which can be compared to 25.3 °C for a F127 solution and 17.6 °C for a P123 solution50 at equivalent concentrations. The variation in the values reflects the difference in the hydrophobic/hydrophilic (PPO/PEO) composition balance. Furthermore, for the same concentration of the two studied block copolymers (P65 and F127), the longer F127 copolymer had both a lower Tonset and a lower Tm in accordance with literature stating that polymers with a larger hydrophobic block, and high-molecular weight polymers of comparable PPO/PEO composition ratio, form micelles at lower temperatures.60
A marked growth of the P65 micelles was revealed at 70 °C and above prior to the phase separation at 82 °C (data not shown). This growth could be a micellar sphere-to-rod transition, which has been found in other PEO–PPO–PEO block copolymer systems. Its origin is related to the increasing core radius with increasing temperature caused by an entropically costly stretching of the PPO blocks and/or to a large mixing of PEO and PPO inside the core with a resulting increase in chemical potential.18,75,76 On the other hand, the observed increase in the cluster radius with temperature below CMT (Fig. 2) was probably caused by difficulties analysing the intensity correlation function close to the base line due to the low light scattering intensity (see below). This was also reflected in the fact that the relaxation mode was broad (Fig. S1b, ESI†).
Static light scattering measurements were performed simultaneously with the DLS measurements. Fig. S2, ESI,† shows the temperature dependence of the light scattering intensity given as the total Rayleigh ratio at the scattering angle θ = 90° (Rθ=90°). The intensity was constant with increasing temperature until it showed a marked increase, which was interpreted as the start of the formation of micelles. A CMT of 40 °C was estimated from the breakpoint in the curve and it corresponded well with the value obtained from DSC (38.1 °C). It should be recalled that the relaxation mode connected to the micelles was observed already at 30 °C (Fig. S1b, ESI†). This was expected since the micellization took place within a certain temperature range, which was due to the gradual decrease in solubility (or polarity) of PPO, as mentioned above, in addition to the polydispersity of the polymer.74,77 Different techniques monitor different aspects of the process.14 It may influence both the amount of unimers in equilibrium with the micelles,78 and the temperature-dependence of the micellar aggregation number.72
Effect of NaGDC on the self-association of P65 and F127
Fig. 3b displays the DSC thermograms for a 1.0 wt% F127 solution with NaGDC at MR = 0–12. A slight decrease in the onset temperature was observed with an increasing amount of NaGDC (Table S4, ESI†). Apparently, the effect of NaGDC on the F127 aggregation was not as prominent as for the P65 aggregation. By comparing the interaction behaviour of the three copolymers with NaGDC, we observed that the micellization of P65 and of F127 was promoted at low NaGDC additions. On the other hand, no induced micelle formation was found for P123 (Fig. 3c).50 The lowering of CMT has previously been observed for several PEO–PPO–PEO block copolymers when mixed with for instance sodium dodecyl sulfate (SDS), which like NaGDC is an anionic surfactant, or cationic surfactants like tetradecyl or dodecyltrimethylammonium bromide (TTAB and DTAB, respectively).14,23,26,27,35,61,80 In the case of the F127–SDS system, Tonset decreased to a minimum at small SDS additions, whereafter a sharp increase could be observed as more SDS was added to the system.26,61 The reason for the initial lowering of the CMT (and below the CMC but above the critical aggregation concentration of the surfactant) originates with the formation of mixed micelles (or complexes).27 Hence, the favourable interactions between the surfactant and copolymer and a synergistic behaviour occurred. As demonstrated by many groups, the signal in the DSC thermograms vanishes at high additions of conventional ionic surfactants to a PEO–PPO–PEO copolymer solution above CMT because the mixed core–shell complexes are broken up and new types of aggregates are formed, see ref. 24, and the references therein, in addition to ref. 35 and 80.
We have recently investigated in detail the different complexes that form in the P123–NaGDC system during the disintegration process of P123 micelles. It was found that in a specific interval of molar ratios, large P123 micelle–NaGDC complexes coexist with small NaGDC micelles with one or several P123 unimers included (“NaDGC–P123” complexes).50 We developed a simplified model to explain the decrease in transition enthalpy as due to an effect of the complex formation (above CMC of NaGDC) of a small bile salt-rich complex comprised by a NaGDC micelle containing one or several block copolymer unimers. It was likely that NaGDC influenced the self-assembly of P65 and F127 in a similar way. In the P65–NaGDC case, no main endothermic signal was detected in the DSC scan at MR = 12 (Fig. 3a and S3a, ESI†). This implied that NaGDC had suppressed the ability for the system to form mixed complexes of core–shell structure in the solution since the signal was correlated with the amount of PPO that dehydrates in the aggregation process. However, as may be observed in Fig. S3a, ESI† for MR = 12 there is a broad “bump” in the scan at temperatures around 20 °C. This could be related to the formation of an NaGDC-rich complex in which the P65 unimer is partly dehydrated as described above. This conclusion was based on the fact that the copolymer chain dehydrated to a minor extent in the interaction with the bile salt aggregate. In the case of the F127–NaGDC and the P123–NaGDC systems, the micellar complexes were still formed at MR = 12 upon increasing temperature, however to a lesser extent as the DSC peak and the corresponding ΔHtr were significantly lowered (see Fig. 3b and c and Table S4, ESI†).50
For all mixed systems, a shift of the relaxation mode related to the complexes towards shorter relaxation times was observed with increasing molar ratios up to MR = 0.6. In this regime, for an increasing molar ratio, there was an increasingly large fraction of GDC− anions that associated with the PEO–PPO–PEO micelles, and hence their overall charge increased. As a consequence the repulsive interparticle interactions increased, which was demonstrated in a fastening of the diffusive translational motion of the complexes. In the F127 case, the influence of interactions on this motion was not as evident as in the cases of P65 and P123 (Fig. 4).
The bimodal distributions at the highest molar ratios, which is clearly observed for the P65 samples in Fig. 4, demonstrated that two diffusing species existed simultaneously; a small NaGDC–P65 complex rich in NaGDC, together with the P65 micelle–NaGDC complex. The behaviour was emphasized for P65–NaGDC mixed solutions containing 5.0 wt% of P65 (Fig. S4, ESI†). At the same copolymer concentration, a bimodal distribution was observed also in the case of F127 at the highest molar ratio studied (Fig. S5, ESI†). Similarly as for classical surfactants with a charged headgroup and a hydrophobic tail,26,28,32,33 bile salts are able to disrupt micelles of PEO–PPO–PEO copolymers to form different types of complexes.50 The broadening of the peaks upon addition of NaGDC may be caused by the formation of the small NaGDC-rich complexes together with the breakup of the P65 micelles as well as the increased uncertainty of the Laplace inversion due to the low light scattering intensity at higher molar ratios. When comparing the distribution at MR = 12 to that of 96 mM NaGDC in water,50 we noticed that there was good agreement between the fast mode and that of the bile salt micelles; the NaGDC-rich complexes were slightly larger possibly due to the incorporation of one or a few block copolymer chains as established before in the P123–NaGDC system.
When comparing the three copolymers and the effect of bile salt on the PEO–PPO–PEO self-assembly it is valuable to discuss this in terms of the number of PO units and EO units in the block copolymer, i.e., its structure and total molecular weight. From the results of the DLS and DSC experiments, we discovered that the micelle of P65 was the easiest to disintegrate. In comparison, the micelles of F127 and P123 behaved similar and were more difficult to disintegrate (see the monomodal relaxation time distributions in Fig. 4). The order of the micelles to resist disintegration (P123 ≈ F127 > P65) is however completely different than that based on the hydrophobic/hydrophilic balance, expressed as a PPO/PEO composition ratio, which decreases in the order: P123 (1.7) > P65 (0.76) > F127 (0.36). We noticed instead that the rank of resistance to disruption was better related to the CMT, which increases in the order: P123 < F127 ≪ P65 (Fig. 3).
This order represents the tendency of the block copolymer to form micelles and it is reasonably connected with the micelle stability. As mentioned before, beside the PPO/PEO ratio, the polymer molecular weight and length of the PPO block are expected to crucially affect the order of the CMTs; the copolymers of higher molecular weight or with large PPO domains showing lower CMT. We observed an intermediate CMT value for F127 because, despite possessing the lowest hydrophobicity, it presented the highest molecular weight and the longest PPO block (the same length as that of P123). Therefore, a slightly higher resistance to disintegration would be expected for the P123 micelles with the respect to the F127 micelles on the basis the order of the CMTs. However, this difference was not observed experimentally when examining the corresponding analyzed relaxation time distributions (Fig. 4). We believe that the presence of the long PEO chains in the corona of the F127 micelles could be an additional stabilizing effect in that it could limit the accessibility of the NaGDC molecules to the core and the consequent PPO block solubilisation.
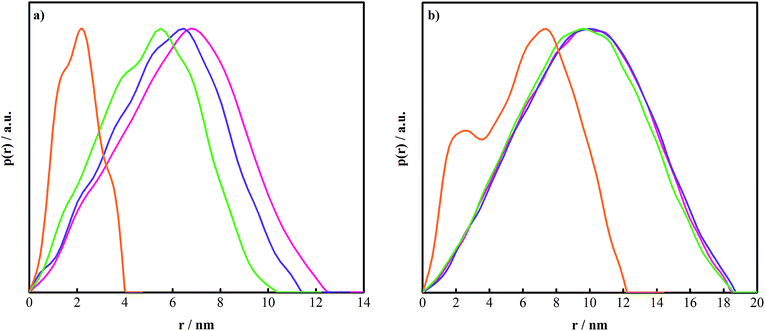
SAXS experiments were carried out at 50 °C on 5.0 wt% PEO–PPO–PEO block copolymer solutions at different molar ratios in the range 0–12.50 The data were analysed by an indirect Fourier transformation (IFT) method by Hansen82 to provide the p(r) functions displayed in Fig. 5 and in ref. 50. The maximum radius, Rmax was estimated as half the maximum dimension of the particles inferred as the distance where the p(r) vanishes. This value is reported in Table 1 along with the radius of gyration, Rg obtained by integrating the p(r) functions. In the case of P65, we observed that Rmax decreased with increasing amount of NaGDC. The data were expected to assay the P65 micelles–NaGDC complexes at low MR (≤0.6) where a slight reduction of the size was induced by the progressive association of NaGDC to the P65 micelles.
| MR | P65–NaGDC | F127–NaGDC | ||
|---|---|---|---|---|
| Rmax/nm | Rg/nm | Rmax/nm | Rg/nm | |
| 0 | 6.1 | 4.8 | 9.1 | 7.3 |
| 0.3 | 5.6 | 4.4 | 9.3 | 7.3 |
| 0.6 | 5.1 | 3.8 | 9.2 | 7.1 |
| 3.0 | 3.2 | 2.4 | 9.1 | 6.7 |
| 12 | 2.0 | 1.6 | 6.0 | 4.7 |
By contrast, at MR = 12, where only NaGDC-rich P65–NaGDC complexes were measured, a significant decrease in size was observed (Fig. 5). Both species were expected to affect the SAXS data at the intermediate MR value of 3 (see the corresponding relaxation time distribution in Fig. 4). Consequently, the p(r) function (not presented) as well as Rg had to be considered as averages (Table 1).
In addition to the IFT analysis, the scattering of the P65–NaGDC mixed solutions was modeled as a form factor P(q) and a structure factor S(q). In the case of the uncharged P65 micelles (MR = 0), a structure factor calculated by using the Percus–Yevick approximation was employed (eqn (S7), ESI†).
At MR > 0, where charged complexes were formed, a structure factor calculated by the Hayter–Penfold (HP) method was instead used in the fitting procedure.83 The P(q) based on the core–shell model (eqn (S6), ESI†) was used at low MR values (≤0.6) to describe the internal structure of the P65 micelle–NaGDC complexes whereas the P(q) of homogeneous spheres (eqn (S8), ESI†) was employed to model the NaGDC–P65 complexes at MR = 12. Fig. 6 shows the selected scattering curves with their fitted curves. The scattering curve of MR = 3 was not possible to evaluate, which was a strong indication that the system contained two types of complexes at this molar ratio.
The output of the data analyses in terms of the core radius Rcore, the shell thickness, dshell and total radius Rtot (= Rcore + dshell) of the complex is summarised in Table S6, ESI.† As can be seen in the table, the Rtot values followed the same decreasing trend as those of Rmax up to MR = 0.6. Furthermore, the Rtot values were overall slightly smaller. The same result was achieved for the micellar complexes in the P123–NaGDC system.51 Rcore of the P65 micellar complexes decreased from 3.5 to 2.8 nm and the shell thickness from 1.9 to 1.2 nm as the molar ratio increased from 0 to 0.6.
The analysed scattering length density (SLD) of the core remained rather constant in the same interval with only a very minor increase (from 9.34 × 10−6 to 9.37 × 10−6 Å−2) while there was a pronounced increase in the SLD of the shell (9.66 × 10−6 to 10.09 × 10−6 Å−2). The same behaviour was also found in the case of the P123–NaGDC system, although in a more extended range of molar ratios. This was interpreted as an indication that the added NaGDC molecules preferentially associated with the shell of the P123 micelles, close to the core/corona interface.51 At MR = 12, the large complexes were to a large extent broken up and only small NaGDC–P65 complexes contributed to the scattering intensity and the form factor of a homogeneous sphere with a SLD of 10.1 × 10−6 Å−2 fitted the data well. The total radius of these complexes was 2.0 nm. This size is similar to that of a pure NaGDC micelle, which will be discussed below.
In the case of the F127–NaGDC system, it was not possible to fit the SAXS data using a core–shell form factor and the HP structure factor. As opposed to P65, F127 had got very long PEO side chains (97 EO units). Because of this, the F127 micelle displayed a star-like structure, i.e., a micelle with a spherical core and a corona composed of Gaussian chains attached to the surface of the core. Hence, a more advanced form factor was required together with an effective structure factor in the analysis of such micelles.84,85 We chose not to fit the data in that manner since the internal structure and the interparticle interference effects were expected to become even more complicated when the bile salt molecules associated to the micelle. The data was therefore only treated by IFT analysis, see ESI.†
At a copolymer concentration of 5.0 wt%, P65 and F127 were unassociated and dissolved in water as single chains at 20 °C, which is <Tonset according to the calorimetric measurements, see Tables S3 and S5, ESI,† respectively. In order to investigate the formation of complexes between unimers and NaGDC at 20 °C, SAXS measurements were performed on the pure copolymers and on mixed solutions at MR = 12.21
In agreement with the presence of unimers, the SAXS curves of the pure F127 and P65 solutions could be fitted to the Debye function of a Gaussian chain86 (eqn (S5), ESI†). This gave a polymer radius of gyration of 2.1 nm and 2.2 nm, respectively (Fig. 7b and c). The intermolecular interactions were negligible and no structure factor was needed in the fits. A Rg value of 2.2 nm for the F127 unimer has been previously reported by Mortensen et al.18,59 Although F127 is a much longer polymer than P65, the two polymers have the same Rg. It could thus be anticipated that, being close to but still below the temperature for the onset of aggregation (21.5 °C, Table S5, ESI†), the F127 unimer shielded its central PPO block by wrapping the PEO chains around it in a similar structure as that of a unimolecular micelle.59,62
When NaGDC was present in the F127 solution at a molar ratio of 12, there occurred a marked change in the SAXS curve, which approached that of the 200 mM NaGDC solution (Fig. 7b) and, at the same time, the effects of the interactions increased at low q values. The curve was fitted to a curve calculated using the form factor of a homogeneous hard sphere and the HP structure factor. A radius Rtot of 1.8 nm was retrieved from the fit, which was the same as that of the NaGDC micelle. The SAXS curve of the mixed system at MR = 12 completely overlapped the curve of the bile salt micelles in the case of P65 whereas some differences were visible in the case of F127. This was reasonable since, at MR = 12, the concentration of NaGDC was closer to that of the pure NaGDC solution in the case of P65 (176 mM) whereas it was significantly lower (47.5 mM) in the case of F127.
Li et al. have investigated the mechanism of formation of SDS micellar aggregates on single F127 chains. In ref. 25 and 26 they discuss how the polymer interacts with the surface of the SDS micelle that is bound to the polymer. This interaction involves the removal of bound water from the micellar surface and from the hydrated parts of the F127 chain. The bound surfactant micelles increase in size and number with the addition of surfactant until the polymer chain become fully saturated.26 Two SANS investigation deals with the same topic albeit on a different PEO–PPO–PEO/SDS system.35,41
According to the MR value, only 12 NaGDC molecules were expected to interact with each block copolymer unimer in our mixtures. This number was within the range of the aggregation number of a NaGDC micelle in water. Based on this, we could rule out the hypothesis that complexes formed by single block copolymer unimers with micelles distributed along the chain were created. Instead, it is suggested that only one small globular aggregate of NaGDC interacted with the F127 unimer forming a NaGDC–F127 complex of similar size to that of a pure NaGDC micelle. The NaGDC molecules most likely interacted with the PPO block in order to partly shield the block from the aqueous media while the rest of the polymer wrapped itself around the NaGDC aggregate. This way, the unimers were prevented from forming micellar complexes with NaGDC as the temperature was raised, see the corresponding DSC scan in Fig. S3b, ESI.† Interestingly, a number of 12 may also be compared to the reported values of about 4–10 SDS molecules being needed to saturate the F127 chain and prevent (or alternatively disrupt) the formation of F127 micelles in the same copolymer concentration regime as the one studied here.23,33,61
The SAXS curves at 20 °C were also collected for the P123–NaGDC system at MR = 0 and 12. According to the DSC data of a 5.0 wt% P123 solution (Fig. S3c, ESI†), 20 °C is above the CMT and P123 self-assembles into micelles under these conditions. Thus, a comparison of data of the two MR values monitored the effect of NaGDC on the P123 micelles. Indeed, as noticed in Fig. 7a, the SAXS curve of the pure P123 solution corresponded to micelles. It was fitted using a core–shell form factor and a hard sphere structure factor (eqn (S6) and (S7) in ESI,† respectively), giving a total radius of the micelle, Rtot, of 7.51 nm. This value was smaller than that previously determined at 40 and 50 °C for the same polymer batch.50,51 Moreover, 7.1 nm was obtained in a recent SANS study of 5.0 wt% P123 in water at 25 °C.40 A marked change was observed in the scattering curve when the solution also contained 104 mM NaGDC (Fig. 7a), again approaching the pattern of the pure NaGDC solution. Indeed the curve of the mixed system, fitted using the form factor of a hard sphere and the HP structure factor generated a Rtot value of 2.0 nm, i.e. the same value as for a NaGDC micelle. The data confirmed that the P123 micelles disintegrated with the addition of NaGDC and bile salt micelles including block copolymer unimers are formed because of disintegration as previously suggested by employing DSC measurements on the same system.50
Conclusions
In the present study, we have systematically investigated three different PEO–PPO–PEO block copolymers, with different molecular weight and block length ratios, and their interaction with the same bile salt. Marked differences between the block copolymers were found, which drive to general conclusions that probably can be extended to conventional surfactants.The PEO–PPO–PEO block copolymers P65, P123 and F127 formed matched pairs in terms of either the length of the PPO or the PEO blocks. They formed micelles at a critical micelle temperature, which was related to the relative block length but also on the molecular weight of the copolymer and the total length of the PPO block. When comparing F127 and P65, F127, which had a higher molecular weight, has a lower CMT although its PPO/PEO composition balance was lower than for P65. The DSC scans revealed that the addition of NaGDC to the F127 and P65 micellar solutions at first lowered the CMT, indicating a synergy effect when the two species were mixed. This effect was different in the case of P123, where the bile salt did not promote micellization of the copolymer. The NaGDC molecules associated to the copolymer micelle, thus forming a charged mixed micellar complex. For all three copolymers, the complex had a core of PPO and a corona of PEO chains, which in the case of P65 and P123 could be modelled as a core–shell internal structure according to the SAXS data. As demonstrated in both light- and X-ray scattering experiments, the micellar complexes experienced an increased repulsive interparticle interaction as they received additional negative charge due to the association of bile salt molecules to the core/corona region. At a constant temperature, the P65 micelle–NaGDC complex was the easiest to break up by the bile salt whereas the complexes with F127 or P123 were only moderately affected. This demonstrated that the ability of the bile salt to disintegrate the block copolymer micelles depended on different factors including hydrophobicity, molecular weight of the polymer and length of the PPO block. It was found that the disintegration of the copolymer micelles followed the same trend as the CMT values of the copolymers. The micellar complexes decreased in size during the disintegration process, which involved the formation of a small bile salt-rich complex that coexisted with the copolymer-rich complex up to high bile salt concentrations (Scheme 2). This second type of complex was pictured as a small spherical aggregate of NaGDC that interacted with one copolymer chain that in turn wrapped around the whole aggregate. This way, the unimers were prevented from forming micellar complexes with dehydrated PPO cores as the temperature was raised.
 | ||
| Scheme 2 Mechanism of disintegration of PEO–PPO–PEO micelles by bile salts (PEO in blue and PPO in red). | ||
This study demonstrated that the self-assembly properties of the mixtures of PEO–PPO–PEO copolymers and natural-human bile salt surfactants can be modulated and significantly be influenced by the features of the copolymeric component. This information is essential for the choice of PEO–PPO–PEO copolymers to be used in novel medical applications involving interactions with bile salts. A possible new application is the use of these copolymers in the treatment of bile-salt related deceases. The idea is that the block copolymer micelles, which are orally administrated, can take up and transport an excess concentration of bile salts out of the body. Another new application is to use mixed micelles of PEO–PPO–PEO block copolymers and bile salt as compartments for delivering a drug by means of transdermal administration to a specific region in the body where the drug is released. The advantage of using a mixed micellar system is that an increased uptake of the drug is anticipated because of the introduction of drug-bile salt intermolecular interactions.
Acknowledgements
We wish to thank S. Lages, beamline scientist at the I911-SAXS beamline, MAX IV Laboratory, Lund, Sweden as well as M. Gubitosi and A. Del Giudice for valuable help during the SAXS measurements. We are grateful to P. Štěpánek, Institute of Macromolecular Chemistry, Prague, Czech Republic, for generously providing us with the REPES program. K. S. acknowledges the Swedish Research Council (VR) (621-2013-4339) and the Linnaeus grant “Organizing Molecular Matter” (239-2009-6749) through VR for financial support and The Knut and Alice Wallenberg Foundation for the general instrument support to the Division of Physical Chemistry. This work has benefited from the SasView software, originally developed by the DANSE project under NSF award DMR-0520547 and from the “Bayesapp” software developed by S. Hansen.Notes and references
- G. Riess, Micellization of Block Copolymers, Prog. Polym. Sci., 2003, 28, 1107–1170 CrossRef CAS.
- F. S. Bates and G. H. Fredrickson, Block Copolymer Thermodynamics: Theory and Experiment, Annu. Rev. Phys. Chem., 1990, 41, 525–557 CrossRef CAS PubMed.
- K. Flodström and V. Alfredsson, Influence of the Block Length of Triblock Copolymers on the Formation of Mesoporous Silica, Microporous Mesoporous Mater., 2003, 59, 167–176 CrossRef.
- E. V. Batrakova and A. V. Kabanov, Pluronic Block Copolymers: Evolution of Drug Delivery Concept from Inert Nanocarriers to Biological Response Modifiers, J. Controlled Release, 2008, 130, 98–106 CrossRef CAS PubMed.
- P. Alexandridis and B. Lindman, Amphiphilic Block Copolymers: Self-Assembly and Applications, Elsevier Science, BV, Amsterdam, 2000 Search PubMed.
- B. Lindman, B. Medronho and G. Karlström, Clouding of Nonionic Surfactants, Curr. Opin. Colloid Interface Sci., 2016, 22, 23–29 CrossRef CAS.
- G. Karlström, A New Model for Upper and Lower Critical Solution Temperatures in Poly(ethylene oxide) Solutions, J. Phys. Chem., 1985, 89, 4962–4964 CrossRef.
- P. Linse, Micellization of Poly(ethylene oxide)–Poly(propylene oxide) Block Copolymers in Aqueous Solution, Macromolecules, 1993, 26, 4437–4449 CrossRef CAS.
- P. Linse, Phase Behavior of Poly(ethylene oxide)–Poly(propylene oxide) Block Copolymers in Aqueous Solution, J. Phys. Chem., 1993, 97, 13896–13902 CrossRef CAS.
- G. Karlström, On the origin of the solution behaviour of ethyleneoxide containing polymers, in Amphiphilic Block Copolymers: Self-Assembly and Applications, ed. Alexandridis, P. and Lindman, B., Elsevier Science, BV, Amsterdam, 2000, pp. 41–55 Search PubMed.
- W. Brown, K. Schillén, M. Almgren, S. Hvidt and P. Bahadur, Micelle and Gel Formation in a Poly(ethylene oxide)–Poly(propylene oxide)–Poly(ethylene oxide) Triblock Copolymer in Water Solution. Dynamic and Static Light Scattering and Oscillatory Shear Measurements, J. Phys. Chem., 1991, 95, 1850–1858 CrossRef CAS.
- K. Schillén, K. Bryskhe and Y. S. Mel’nikova, Vesicles Formed from a Poly(ethylene oxide)–Poly(propylene oxide)–Poly(ethylene oxide) Triblock Copolymer in Dilute Aqueous Solution, Macromolecules, 1999, 32, 6885–6888 CrossRef.
- G. Wanka, H. Hoffman and W. Ulbricht, Phase Diagrams and Aggregation Behavior of Poly(oxyethylene)–Poly(oxypropylene)–Poly(oxyethylene) Triblock Copolymers in Aqueous Solutions, Macromolecules, 1994, 27, 4145–4159 CrossRef CAS.
- M. Almgren, W. Brown and S. Hvidt, Self-Aggregation and Phase Behavior of Poly(ethylene oxide)–Poly(propylene oxide)–Poly(ethylene oxide) Block Copolymers in Aqueous Solution, Colloid Polym. Sci., 1995, 273, 2–15 CAS.
- P. Alexandridis and T. A. Hatton, Poly(ethylene oxide)–Poly(propylene oxide)–Poly(ethylene oxide) Block Copolymers Surfactants in Aqueous Solutions and at Interfaces: Thermodynamics, Structure, Dynamics and Modeling, Colloids Surf., A, 1995, 96, 1–46 CrossRef CAS.
- B. Chu and Z. Zhou, Physical chemistry of polyoxyalkylene block copolymer surfactants, in Nonionic Surfactants: Polyoxyalkylene Block Copolymers, ed. Nace, V. M., Marcel Dekker, Inc., 1996, vol. 60, pp. 67–144 Search PubMed.
- P. Alexandridis, Poly(ethylene oxide)/Poly(propylene oxide)/Poly(ethylene oxide) Block Copolymer Surfactants, Curr. Opin. Colloid Interface Sci., 1997, 2, 478–489 CrossRef CAS.
- K. Mortensen, Structural Studies of Aqueous Solutions of PEO–PPO–PEO Triblock Copolymers, their Micellar Aggregates and Mesophases: A Small-Angle Neutron Scattering Study, J. Phys.: Condens. Matter, 1996, 8, A103–A124 CrossRef CAS.
- K. Mortensen, PEO-Related Block Copolymer Surfactants, Colloids Surf., A, 2001, 183–185, 277–292 CrossRef CAS.
- S. Manet, A. Lecchi, M. Imperor-Clerc, V. Zholobenko, D. Durand, C. L. P. Oliveira, J. S. Pedersen, I. Grillo, F. Meneau and C. Rochas, Structure of Micelles of a Nonionic Block Copolymer Determined by SANS and SAXS, J. Phys. Chem. B, 2011, 115, 11318–11329 CrossRef CAS PubMed.
- K. Schillén, J. Jansson, D. Löf and T. Costa, Mixed Micelles of a PEO–PPO–PEO Triblock Copolymer (P123) and a Nonionic Surfactant (C12EO6) in Water. A Dynamic and Static Light Scattering Study, J. Phys. Chem. B, 2008, 112, 5551–5562 CrossRef PubMed.
- M. Almgren, J. van Stam, C. Lindblad, P. Li, P. Stilbs and P. Bahadur, Aggregation of Poly(ethylene oxide)–Poly(propylene oxide)–Poly(ethylene oxide) Triblock Copolymers in the Presence of Sodium Dodecyl Sulfate in Aqueous Solution, J. Phys. Chem., 1991, 95, 5677–5684 CrossRef CAS.
- E. Hecht and H. Hoffmann, Interaction of ABA Block Copolymers with Ionic Surfactants in Aqueous Solution, Langmuir, 1994, 10, 86–91 CrossRef CAS.
- N. V. Sastry and H. Hoffman, Interaction of Amphiphilic Block Copolymer Micelles with Surfactants, Colloids Surf., A, 2004, 250, 247–261 CrossRef CAS.
- Y. Li, R. Xu, D. M. Bloor, J. F. Holzwarth and E. Wyn-Jones, The Binding of Sodium Dodecyl Sulfate to the ABA Block Copolymer Pluronic F127 (EO97PO69EO97): An Electromotive Force, Microcalorimetry, and Light Scattering Investigation, Langmuir, 2000, 16, 10515–10520 CrossRef CAS.
- Y. Li, R. Xu, S. Couderc, D. M. Bloor, E. Wyn-Jones and J. F. Holzwarth, The Binding of Sodium Dodecyl Sulfate to the ABA Block Copolymer Pluronic F127 (EO97PO69EO97): F127 Aggregation Induced by SDS, Langmuir, 2001, 17, 183–188 CrossRef CAS.
- Y. Li, R. Xu, S. Couderc, D. M. Bloor, J. F. Holzwarth and E. Wyn-Jones, Binding of Tetradecyltrimethylammonium Bromide to the ABA Block Copolymer Pluronic F127 (EO97PO69EO97): Electromotive Force, Microcalorimetry, and Light Scattering Studies, Langmuir, 2001, 17, 5742–5747 CrossRef CAS.
- J. Jansson, K. Schillén, G. Olofsson, R. C. da Silva and W. Loh, The Interaction between PEO–PPO–PEO Triblock Copolymers and Ionic Surfactants in Aqueous Solution Studied using Light Scattering and Calorimetry, J. Phys. Chem. B, 2004, 108, 82–92 CrossRef CAS.
- A. H. Roux, G. Douhéret and G. Roux-Desgranges, Molar Volumes and Isentropic Compressions of Pluronics L64 and P123 in Aqueous Surfactant Solutions, over the Critical Temperature Range of Aggregation, Colloids Surf., A, 2005, 252, 43–50 CrossRef CAS.
- M. Kumbhakar, Aggregation of Ionic Surfactants to Block Copolymer Assemblies: A Simple Fluorescence Spectral Study, J. Phys. Chem. B, 2007, 111, 14250–14255 CrossRef CAS PubMed.
- R. Wang, Y. Tang and Y. Wang, Effects of Cationic Ammonium Gemini Surfactant on Micellization of PEO–PPO–PEO Triblock Copolymers in Aqueous Solution, Langmuir, 2014, 30, 1957–1968 CrossRef CAS PubMed.
- J. Jansson, K. Schillén, M. Nilsson, O. Söderman, G. Fritz, A. Bergmann and O. Glatter, Small-Angle X-ray Scattering, Light Scattering, and NMR Study of PEO–PPO–PEO Triblock Copolymer/Cationic Surfactant Complexes in Aqueous Solution, J. Phys. Chem. B, 2005, 109, 7073–7083 CrossRef CAS PubMed.
- E. Hecht, K. Mortensen, M. Gradzielski and H. Hoffmann, Interaction of ABA Block Copolymers with Ionic Surfactants: Influence of Micellization and Gelation, J. Phys. Chem., 1995, 99, 4866–4874 CrossRef CAS.
- T. Thurn, S. Couderc, J. Sidhu, D. M. Bloor, J. Penfold, J. F. Holzwarth and E. Wyn-Jones, Study of Mixed Micelles and Interaction Parameters for ABA Triblock Copolymers of the type EOm–POn–EOm and Ionic Surfactants: Equilibrium and Structure, Langmuir, 2002, 18, 9267–9275 CrossRef CAS.
- S. Couderc-Azouani, J. Sidhu, T. Thurn, R. Xu, D. M. Bloor, J. Penfold, J. F. Holzwarth and E. Wyn-Jones, Binding of Sodium Dodecyl Sulfate and Hexaethylene Glycol Mono-n-Dodecyl Ether to the Block Copolymer L64:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Electromotive Force, Microcalorimetry, Surface Tension, and Small Angle Neutron Scattering Investigations of Mixed Micelles and Polymer/Micellar Surfactant Complexes, Langmuir, 2005, 21, 10197–10208 CrossRef CAS PubMed.
Electromotive Force, Microcalorimetry, Surface Tension, and Small Angle Neutron Scattering Investigations of Mixed Micelles and Polymer/Micellar Surfactant Complexes, Langmuir, 2005, 21, 10197–10208 CrossRef CAS PubMed. - J. B. Vieira, R. K. Thomas, Z. X. Li and J. Penfold, Unusual Micelle and Surface Adsorption Behavior in Mixtures of Surfactants with an Ethylene Oxide–Propylene Oxide Triblock Copolymer, Langmuir, 2005, 21, 4441–4451 CrossRef CAS PubMed.
- R. Ganguly, V. K. Aswal, P. A. Hassan, I. K. Gopalakrishnan and S. K. Kulshreshtha, Effect of SDS on the Self-Assembly Behavior of the PEO–PPO–PEO Triblock Copolymer (EO)20(PO)70(EO)20, J. Phys. Chem. B, 2006, 110, 9843–9849 CrossRef CAS PubMed.
- P. K. Singh, M. Kumbhakar, R. Ganguly, V. K. Aswal, H. Pal and S. Nath, Time-Resolved Fluorescence and Small Angle Neutron Scattering Study in Pluronics-Surfactant Supramolecular Assemblies, J. Phys. Chem. B, 2010, 114, 3818–3826 CrossRef CAS PubMed.
- J. S. Nambam and J. Philip, Effects of Interaction of Ionic and Nonionic Surfactants on Self-Assembly of PEO–PPO–PEO Triblock Copolymer in Aqueous Solution, J. Phys. Chem. B, 2012, 116, 1499–1507 CrossRef CAS PubMed.
- O. T. Mansour, B. Cattoz, R. K. Heenan, S. M. King and P. C. Griffiths, Probing Competitive Interactions in Quaternary Formulations, J. Colloid Interface Sci., 2015, 454, 35–43 CrossRef CAS PubMed.
- G. K. S. Prameela, B. V. N. Phani Kumar, A. Pan, V. K. Aswal, J. Subramanian, A. B. Mandal and S. P. Moulik, Physico-Chemical Perspectives (Aggregation, Structure and Dynamics) of Interaction between Pluronic (L31) and Surfactant (SDS), Phys. Chem. Chem. Phys., 2015, 17, 30560–30569 RSC.
- A. F. Hofmann and K. Mysels, Bile Salts as Biological Surfactants, Colloids Surf., 1987, 30, 145–173 CrossRef.
- S. Mukhopadhyay and U. Maitra, Chemistry and Biology of Bile Acids, Curr. Sci., 2004, 87, 1666–1683 CAS.
- D. Madenci and S. U. Egelhaaf, Self-Assembly in Aqueous Bile Salt Solutions, Ther. Adv. Gastroenterol., 2010, 15, 109–115 CAS.
- L. Galantini, M. C. di Gregorio, M. Gubitosi, L. Travaglini, J. V. Tato, A. Jover, F. Meijide, V. H. Soto Tellini and N. V. Pavel, Bile Salts and Derivatives: Rigid Unconventional Amphiphiles as Dispersants, Carriers and Superstructure Building Blocks, Curr. Opin. Colloid Interface Sci., 2015, 20, 170–182 CrossRef CAS.
- N. S. Cameron, A. Eisenberg and G. R. Brown, Amphiphilic Block Copolymers as Bile Acid Sorbents: 1. Synthesis of Polystyrene-b-poly(N,N,N-trimethylammoniumethylene acrylamide chloride), Biomacromolecules, 2002, 3, 116–123 CrossRef CAS PubMed.
- N. S. Cameron, A. Eisenberg and G. R. Brown, Amphiphilic Block Copolymers as Bile Acid Sorbents: 2. Polystyrene-b-poly(N,N,N-trimethylammoniumethylene acrylamide chloride): Self-Assembly and Application to Serum Cholesterol Reduction, Biomacromolecules, 2002, 3, 124–132 CrossRef CAS PubMed.
- P. Zarras and O. Vol, Polycationic Salts as Bile Acid Sequestering Agents, Prog. Polym. Sci., 1999, 24, 485–516 CrossRef CAS.
- J. R. F. Walters and S. S. Pattni, Managing Bile Acid Diarrhoea, Ther. Adv. Gastroenterol., 2010, 3, 349–357 CrossRef PubMed.
- S. Bayati, L. Galantini, K. D. Knudsen and K. Schillén, Effects of Bile Salt Sodium Glycodeoxycholate on the Self-Assembly of PEO–PPO–PEO Triblock Copolymer P123 in Aqueous Solution, Langmuir, 2015, 31, 13519–13527 CrossRef CAS PubMed.
- S. Bayati, L. Galantini, K. D. Knudsen and K. Schillén, Complexes of PEO–PPO–PEO Triblock Copolymer P123 and Bile Salt Sodium Glycodeoxycholate in Aqueous Solution: a Small Angle X-ray and Neutron Scattering Investigation, Colloids Surf., A, 2016, 504, 426–436 CrossRef CAS.
- A. Roy, N. Kundu, D. Banik, J. Kuchlyan and N. Nilmoni Sarkar, How Does Bile Salt Penetration Affect the Self-Assembled Architecture of Pluronic P123 Micelles? – Light Scattering and Spectroscopic Investigations, Phys. Chem. Chem. Phys., 2015, 17, 19977–19990 RSC.
- D. Löf, A. Niemiec, K. Schillén, W. Loh and G. Olofsson, A Calorimetry and Light Scattering Study of the Formation and Shape Transition of Mixed Micelles of EO20PO68EO20 Triblock Copolymer (P123) and Nonionic Surfactant (C12EO6), J. Phys. Chem. B, 2007, 111, 5911–5920 CrossRef PubMed.
- J. Janiak, S. Bayati, L. Galantini, N. V. Pavel and K. Schillén, Nanoparticles with a Bicontinuous Cubic Internal Structure Formed by Cationic and Non-Ionic Surfactants and an Anionic Polyelectrolyte, Langmuir, 2012, 28, 16536–16546 CrossRef CAS PubMed.
- A. Labrador, Y. Cerenius, C. Svenssson, K. Theodor and T. Plivelic, The Yellow Mini-Hutch for SAXS Experiments at MAX IV Laboratory, in 11th International Conference on Synchrotron Radiation Instrumentation, ed. Dumas, P. S. J., IOP Publishing Ltd, Lyon, France, 2013, p. 072019 Search PubMed.
- D. Attwood, J. H. Collett and C. J. Tait, The Micellar Properties of the Poly(oxyethylene)–Poly(oxypropylene)–Poly(oxyethylene) Copolymer Pluronic F127 in Water and Electrolyte Solution, Int. J. Pharm., 1985, 26, 25–33 CrossRef CAS.
- G. Wanka, H. Hoffman and W. Ulbricht, The Aggregation Behavior of Poly(oxyethylene)–Poly(oxypropylene)–Poly(oxyethylene)-Block-Copolymers in Aqueous Solution, Colloid Polym. Sci., 1990, 268, 101–117 CAS.
- G.-E. Yu, Y. Deng, S. Dalton, Q.-G. Wang, D. Attwood, C. Price and C. Booth, Micellisation and Gelation of Triblock Copoly(oxyethylene/oxypropylene/oxyethylene), F127, J. Chem. Soc., Faraday Trans., 1992, 88, 2537–2544 RSC.
- K. Mortensen and Y. Talmon, Cryo-TEM and SANS Microstructural Study of Pluronic Polymer Solutions, Macromolecules, 1995, 28, 8829–8834 CrossRef CAS.
- P. Alexandridis, J. F. Holzwarth and T. A. Hatton, Micellization of Poly(ethylene oxide)–Poly(propylene oxide)–Poly(ethylene oxide) Triblock Copolymers in Aqueous Solutions: Thermodynamics of Copolymer Association, Macromolecules, 1994, 27, 2414–2425 CrossRef CAS.
- R. C. da Silva, G. Olofsson, K. Schillén and W. Loh, Influence of Ionic Surfactants on the Aggregation of Poly(ethylene oxide)–Poly(propylene oxide)–Poly(ethylene oxide) Block Copolymers Studied by Differential Scanning and Isothermal Titration Calorimetry, J. Phys. Chem. B, 2002, 106, 1239–1246 CrossRef.
- Y.-L. Su, H.-Z. Liu, J. Jing Wang and J.-Y. Chen, Study of Salt Effects on the Micellization of PEO–PPO–PEO Block Copolymer in Aqueous Solution by FTIR Spectroscopy, Langmuir, 2002, 18, 865–871 CrossRef CAS.
- A. E. Beezer, W. Loh, J. C. Mitchell, P. G. Royall, D. O. Smith, M. S. Tute, J. K. Armstrong, B. Z. Chowdhry and S. A. Leharne, An Investigation of Dilute Aqueous Solution Behavior of Poly(oxyethylene) + Poly(oxypropylene) + Poly(oxyethylene) Block Copolymers, Langmuir, 1994, 10, 4001–4006 CrossRef CAS.
- R. Ganguly, V. K. Aswal, P. A. Hassan, I. K. Gopalakrishnan and J. V. Yakhmi, Sodium Chloride and Ethanol Induced Sphere to Rod Transition of Triblock Copolymer Micelles, J. Phys. Chem. B, 2005, 109, 5653–5658 CrossRef CAS PubMed.
- Y. Kadam, R. Ganguly, M. Kumbhakar, V. K. Aswal, P. A. Hassan and P. Bahadur, Time Dependent Sphere-to-Rod Growth of the Pluronic Micelles: Investigating the Role of Core and Corona Solvation in Determining the Micellar Growth Rate, J. Phys. Chem. B, 2009, 113, 16296–16302 CrossRef CAS PubMed.
- A. Bogomolova, M. Hruby, J. Panek, M. Rabyk, S. Turner, S. Bals, M. Steinhart, A. Zhigunov, O. Sedlacek, P. Stepanek and S. K. Filippov, Small-Angle X-ray Scattering and Light Scattering Study of Hybrid Nanoparticles Composed of Thermoresponsive Triblock Copolymer F127 and Thermoresponsive Statistical Polyoxazolines with Hydrophobic Moieties, J. Appl. Crystallogr., 2013, 46, 1690–1698 CrossRef CAS.
- N. J. Jain, A. George and P. Bahadur, Effect of Salt on the Micellization of Pluronic P65 in Aqueous Solution, Colloids Surf., A, 1999, 157, 275–283 CrossRef CAS.
- J. P. Mata, P. R. Majhi, O. Kubota, A. Khanal, K. Nakashima and P. Bahadur, Effect of Phenol on the Aggregation Characteristics of an Ethylene Oxide–Propylene Oxide Triblock Copolymer P65 in Aqueous Solution, J. Colloid Interface Sci., 2008, 320, 275–282 CrossRef CAS PubMed.
- H.-W. Tsui, J.-H. Wang, Y.-H. Hsu and L.-J. Chen, Study of Heat of Micellization and Phase Separation for Pluronic Aqueous Solutions by using a High Sensitivity Differential Scanning Calorimetry, Colloid Polym. Sci., 2010, 288, 1687–1696 CAS.
- B. Chowdhry, M. J. Snowden, C. MacLeod and S. A. Leharne, Identification and Deconvolution of Dissociation and Aggregation Transitions during Thermally Induced Micellization in Aqueous Solutions of Ethylene Oxide–Propylene Oxide–Ethylene Oxide Block Copolymers, Thermochim. Acta, 2000, 359, 29–36 CrossRef CAS.
- http://worldaccount.basf.com/wa/NAFTA%7Een_GB/Catalog/ChemicalsNAFTA/pi/BASF/Subgroup/non_ionic_surfactants/surfactants/productgroup_top.
- L. Chiappisi, G. Lazzara, M. Gradzielski and S. Milioto, Quantitative Description of Temperature Induced Self-Aggregation Thermograms Determined by Differential Scanning Calorimetry, Langmuir, 2012, 28, 17609–17616 CrossRef CAS PubMed.
- W. Brown, K. Schillén and S. Hvidt, Triblock Copolymers in Aqueous Solution Studied by Static and Dynamic Light Scattering and Oscillatory Shear Measurements. Influence of Relative Block Sizes, J. Phys. Chem., 1992, 96, 6038–6044 CrossRef CAS.
- W. Batsberg, S. Ndoni, C. Trandum and S. Hvidt, Effects of Poloxamer Inhomogeneities on Micellization in Water, Macromolecules, 2004, 37, 2965–2971 CrossRef CAS.
- K. Mortensen and W. Brown, Poly(ethylene oxide)–Poly(propylene oxide)–Poly(ethylene oxide) Triblock Copolymers in Aqueous Solution. The Influence of Relative Block Size, Macromolecules, 1993, 26, 4128–4135 CrossRef CAS.
- K. Schillén, W. Brown and R. M. Johnsen, Micellar Sphere-to-Rod Transition in an Aqueous Triblock Copolymer System. A Dynamic Light Scattering Study of Translational and Rotational Diffusion, Macromolecules, 1994, 27, 4825–4832 CrossRef.
- P. Linse, Micellization of Poly(ethylene oxide)–Poly(propylene oxide) Block Copolymers in Aqueous Solution: Effect of Polymer Polydispersity, Macromolecules, 1994, 27, 2685–2693 CrossRef CAS.
- P. Alexandridis and J. F. Holzwarth, Differential Scanning Calorimetry Investigation of the Effect of Salts on Aqueous Solution Properties of an Amphiphilic Block Copolymer (Poloxamer), Langmuir, 1997, 13, 6074–6082 CrossRef CAS.
- K. Nakashima and P. Bahadur, Aggregation of Water-Soluble Block Copolymers in Aqueous Solutions: Recent Trends, Adv. Colloid Interface Sci., 2006, 123–126, 75–96 CrossRef CAS PubMed.
- J. Mata, T. Joshi, D. Varade, G. Ghosh and P. Bahadur, Aggregation Behavior of a PEO–PPO–PEO Block Copolymer + Ionic Surfactants Mixed Systems in Water and Aqueous Salt Solutions, Colloids Surf., A, 2004, 247, 1–7 CrossRef CAS.
- K. Matsuoka, M. Maeda and Y. Moroi, Micelle Formation of Sodium Glyco- and Taurocholates and Sodium Glyco- and Taurodeoxycholates and Solubilization of Cholesterol into Their Micelles, Colloids Surf., A, 2003, 32, 87–95 CAS.
- S. Hansen, Simultaneous Estimation of the Form Factor and Structure Factor for Globular Particles in Small-Angle Scattering, J. Appl. Crystallogr., 2008, 41, 436–445 CrossRef CAS.
- J. H. Hayter and J. Penfold, An Analytic Structure Factor for Macroion Solutions, Mol. Phys., 1981, 42, 109–118 CrossRef CAS.
- J. S. Pedersen and M. C. Gerstenberg, Scattering Form Factor of Block Copolymer Micelles, Macromolecules, 1996, 29, 1363–1365 CrossRef CAS.
- J. S. Pedersen, Structure Factors Effects in Small-Angle Scattering from Block Copolymer Micelles and Star Polymers, J. Chem. Phys., 2001, 114, 2839–2846 CrossRef CAS.
- K. Mortensen and J. S. Pedersen, Structural Study on the Micelle Formation of Poly(ethylene oxide)–Poly(propylene oxide)–Poly(ethylene oxide) Triblock Copolymer in Aqueous Solution, Macromolecules, 1993, 26, 805–812 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: DSC, DLS and SLS data of the P65–water system, DSC data of the block copolymer–NaGDC mixed systems, results of SAXS data analysis of the P65–NaGDC mixed system, description of experimental methods. See DOI: 10.1039/c6ra12514j |
| ‡ Current address: AkzoNobel Industrial Coatings AB, Staffanstorpsvägen 50, SE – 205 17 Malmö, Sweden. |
| This journal is © The Royal Society of Chemistry 2016 |