Solvothermal synthesized LiMn1−xFexPO4@C nanopowders with excellent high rate and low temperature performances for lithium-ion batteries†
Bangkun Zoua,
Ran Yua,
Miaomiao Dengab,
Yuting Zhoua,
Jiaying Liaoa and
Chunhua Chen*a
aCAS Key Laboratory of Materials for Energy Conversions, Department of Materials Science and Engineering & Collaborative Innovation Center of Suzhou Nano Science and Technology, University of Science and Technology of China, Hefei 230026, Anhui, China. E-mail: cchchen@ustc.edu.cn; Fax: +86-551-63601592; Tel: +86-551-63606971
bSchool of Chemistry and Chemical Engineering, HeFei University of Technology, Hefei 230009, Anhui, China
First published on 24th May 2016
Abstract
Mixed-carbon coated LiMn1−xFexPO4 (x = 0, 0.2, 0.5, 1) nano-particles are synthesized by a novel solvothermal approach. All of these powders possess a uniform particle size distribution around 150 nm and a carbon coating layer of about 2 nm. The LiMn1−xFexPO4@C samples with a carbon content of 2 wt% have an optimal electrochemical performance. The average voltage platform of LiMn1−xFexPO4@C increases with the increased Mn/Fe ratio, but declines gradually during electrochemical cycling. The LiMn0.5Fe0.5PO4 sample shows a high energy density (568 W h kg−1), good cycleability (97.1%, 100 cycles) and excellent rate capability (120.2 mA h g−1, 20C) at room temperature. Simultaneously, the LiMn0.5Fe0.5PO4 and LiFePO4 samples also show excellent low temperature electrochemical performance with specific capacities of 109.4 and 138.8 mA h g−1 with average discharge voltages of 3.476 V and 3.385 V, respectively, at −12 °C. Even at −20 °C, their discharge specific capacities are 71.7 and 82.3 mA h g−1 at 3C, respectively.
1. Introduction
A lithium-ion battery (LIB) can reach a high energy density and long cycle life, which gives it remarkable advantages in the field of energy storage systems. In terms of materials for LIBs, olivine-type orthophosphates LiMPO4 (M = Fe, Mn, Co and Ni) are currently the representative cathodes for the applications of electric vehicles.1–4 Among them, LiMnPO4 can deliver a higher energy density than LiFePO4 due to the difference in the higher voltage plateaus against Li/Li+, i.e. 4.0 V for LiMnPO4 vs. 3.4 V for LiFePO4.2,5 The voltage plateaus of LiCoPO4 and LiNiPO4 are 4.8 V and 5.1 V, respectively. However, the usual organic electrolyte systems are electrochemically unstable above 4.8 V. Therefore, LiMnPO4 is considered as the most promising candidate to replace LiFePO4. Unfortunately, it is difficult to achieve a desirable electrochemical performance due to its rather poor intrinsic electronic conductivity and very low lithium ion diffusion coefficient.Just like the previous works on LiFePO4, in order to improve the electrochemical performance of LiMnPO4, it is necessary to introduce a small amount of carbon component such as pyrolytic carbons and graphene to form a composite cathode material.6–11 On the other hand, other strategies such as making nano-particles12,13 and cationic doping (Fe2+, Mg2+, V3+, Cr3+, Zr4+)14–20 are also adopted to improve the electrochemical properties of LiMnPO4. However, too much carbon coating and substitution of Mn2+ with inert metal ions would decrease the specific capacity. Nevertheless, the partial substitution of Mn2+ with Fe2+ in LiMn1−xFexPO4 (LMFP) (0 < x < 1) does not decrease the specific capacity because Fe2+ can be also reversibly changed to Fe3+ within the setting voltage range. Such a substitution can largely increase the electrochemical activity of LMFP. However, what is the optimal Mn/Fe ratio in LMFP electrode is not quite clear. Also, the increase of Mn/Fe ratio usually results in higher theoretical energy density due to its higher voltage plateau, but simultaneously with worse cycling and rate performance.21–23
Herein, a well-designed solvothermal method with a mixed solvent of deionized water and ethylene glycol is employed to synthesize LiMn1−xFexPO4 (x = 0, 0.2, 0.5, 1) nanopowders. In this synthesis process, ascorbic acid is used not only as an antioxidant and surfactant, but also as one of two carbon sources. The composition LiMn0.5Fe0.5PO4 with about 2 wt% carbon content demonstrates optimal electrochemical performance at room and low temperatures.
2. Experimental
2.1 Synthesis of materials
LiMn1−xFexPO4 (x = 0, 0.2, 0.5, 1) cathode materials were synthesized with a novel solvothermal method shown in Fig. 1.24 In brief, 6 mmol LiOH was dissolved in the water–ethylene glycol mixture solvent to obtain a solution A. 2.2 mmol H3PO4 was dissolved in ethylene glycol to obtain a solution B. 0.4 g ascorbic acid was dissolved in the water/ethylene glycol mixed solvent, followed by adding 2(1 − x) mmol MnSO4·4H2O and 2x mmol FeSO4·7H2O to obtain a series of solutions C (x = 0, 0.2, 0.5, 1). Then, a milky white suspension D was obtained by mixing solution A and solution B under vigorous magnetic stirring. Subsequently, the suspension E was obtained by mixing a solution C and the suspension D, and then E was transferred into a 100 mL Teflon-lined stainless steel autoclave and sealed. The autoclave was put into an electric oven and kept at 180 °C for 10 h. Finally, the solvothermal products were collected by centrifuging and washing for several times with deionized water and absolute alcohol.In order to improve the electrical conductivity of the samples, the solvothermal products were mixed with a certain content of glucose (8 wt% and 15 wt% of the solvothermal products) in an agate mortar with some deionized water and ethyl alcohol as the dispersants. After drying, the mixtures were calcined in a tubular furnace at 350 °C for 2 h and subsequently at 650 °C for 8 h under Ar/H2 (5 vol% H2) atmosphere to obtain carbon-coated powders LiMn1−xFexPO4@C (x = 0, 0.2, 0.5, 1). Here we assign D0-LiMn1−xFexPO4, D8-LiMn1−xFexPO4 and D15-LiMn1−xFexPO4 for the samples prepared with 0 wt%, 8 wt% and 15 wt% glucose, respectively.
2.2 Characterization of materials
X-ray powder diffraction (XRD, Rigaku TTR-III, CuKα radiation) was used to characterize the crystal structure of the LMFP samples. Scanning electron microscopy (SEM, JSM-6390 LA, JEOL) and (high resolution) transmission electron microscopy (TEM, HRTEM, JEM-2010) were carried out to analyse their particle morphologies. The specific surface area and the pore size distribution were measured by N2-adsorption/desorption (Tristar II 3020M, Micromeritics). The signal characteristic of the carbon coating was measured by Raman spectroscopy (Renishaw inVia Raman Microscope) on the condition of 532 nm diode laser excitation and the carbon content was analysed by Infrared Carbon-sulfur analyzer (CS-8800C, Jinbo).2.3 Electrochemical measurements
CR2032-type coin-cells were assembled to evaluate the electrochemical performances of the LMFP powders. The as-prepared active materials were mixed with carbon black and polyvinylidene fluoride binder at a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dispersed in N-methyl-2-pyrrolidone to form slurries, which were then casted onto aluminium foils to make working electrodes. A lithium foil was used as the counter electrode, and Celgard 2400 polypropylene membrane as a porous separator. The electrolyte was 1.0 M LiPF6 dissolved in ethylene carbonate (EC) and dimethyl carbonate (DMC) (1
1 dispersed in N-methyl-2-pyrrolidone to form slurries, which were then casted onto aluminium foils to make working electrodes. A lithium foil was used as the counter electrode, and Celgard 2400 polypropylene membrane as a porous separator. The electrolyte was 1.0 M LiPF6 dissolved in ethylene carbonate (EC) and dimethyl carbonate (DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, Zhuhai Smoothway Electronics Materials Co. Ltd). The loading density of active materials was about 2 mg cm−2. The cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) of the cells were performed on a CHI 660B electrochemical workstation. The CV was measured in a voltage range from 2.2 to 4.5 V at a scan rate of 0.1 mV s−1 for LiMn1−xFexPO4 (x = 0, 0.2, 0.5) and 2.2 to 4.0 V for LiFePO4. The cells were also galvanostatically cycled on a multi-channel battery test system (Neware BTS-2300, Shenzhen) in the voltage range from 2.2 to 4.5 V for LiMn1−xFexPO4 (x = 0, 0.2, 0.5) and 2.2 to 4.0 V for LiFePO4 at room temperature (25 ± 2 °C) and low temperatures (−12 °C and −20 °C).
1, v/v, Zhuhai Smoothway Electronics Materials Co. Ltd). The loading density of active materials was about 2 mg cm−2. The cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) of the cells were performed on a CHI 660B electrochemical workstation. The CV was measured in a voltage range from 2.2 to 4.5 V at a scan rate of 0.1 mV s−1 for LiMn1−xFexPO4 (x = 0, 0.2, 0.5) and 2.2 to 4.0 V for LiFePO4. The cells were also galvanostatically cycled on a multi-channel battery test system (Neware BTS-2300, Shenzhen) in the voltage range from 2.2 to 4.5 V for LiMn1−xFexPO4 (x = 0, 0.2, 0.5) and 2.2 to 4.0 V for LiFePO4 at room temperature (25 ± 2 °C) and low temperatures (−12 °C and −20 °C).
3. Results and discussion
3.1 Structures analyses of LMFP nanopowders
As can be seen the synthetic process shown in Fig. 1, the deionized water in the mixed solvent increases the solubility of the reactants. The ethylene glycol acts as a dispersant agent and can prevent the precipitation of the suspension E when the solution C is added dropwise in D. Ascorbic acid is adopted as an antioxidant, surfactant and the first carbon source in the solvothermal process. After mixing the as-solvothermal powders with an appropriate amount of glucose as the second carbon source for a post-heat treatment, mixed-carbon-coated LiMn1−xFexPO4 samples can be synthesized.The XRD patterns of D8-LiMn1−xFexPO4 (x = 0, 0.2, 0.5, 1) are presented in Fig. 2. From Fig. 2a, all diffraction patterns can be indexed as the typical features of olivine-type structure LiFePO4 (PDF # 81-1173) peaks without any impurity phase. It is noted that the diffraction peaks shift toward large angle with gradually decreased Mn/Fe ratio in the samples (Fig. 2b), suggesting the lattice contraction caused by the smaller ionic radius of Fe2+ (76 pm) than that of Mn2+ (80 pm). The preferred orientation can be deduced from the intensity ratio of I020/I200, the higher value of I020/I200 suggests the preferential growth direction along the ac facet (pattern a), and otherwise, it indicates the possible preferred orientation of bc-plane (pattern d).25,26
 | ||
| Fig. 2 XRD patterns of (a–d) which represent D8-LiMn1−xFexPO4 (x = 0, 0.2, 0.5, 1), respectively (a), diffraction angle from 15° to 30° is zoomed (b). | ||
Raman spectroscopy analysis can identify the presence of carbon in the samples (Fig. 3). The peaks at 800–1120 cm−1 are due to the PO43− ions in D8-LMFP, while the two peaks at 1330 cm−1 and 1595 cm−1 can be assigned to the D- and G-bands of carbon, which is obtained from the carbonization of glucose.
3.2 Morphology of LMFP nanopowders
Fig. 4 shows the SEM images of the D8-LMFP samples with different Fe contents. It can be seen that the shape of D8-LiMnPO4 particles look like rectangular square nano-sheets of about 150–200 nm in width and several nanometers in thickness (Fig. 4a). However, with the decrease of Mn/Fe ratio (Fig. 4b–d), the nano-sheets gradually change into short nanorods with a size of 30–50 nm in diameter and 100–150 nm in length. The preferential growth of LMFP crystals in the solvothermal process is considerably affected by the manganese content, which is also consistent with the XRD results (the relative peak intensity of (020) vs. (200) planes). The possible reason is the main Mn2+/Fe2+ ratio in the liquid part of the precursor, it is clearly that the electronegativity of Mn2+ is larger than Fe2+, that means Mn2+ cations show much stronger adsorption on crystal nucleus, and they may act as a template to affect the growth speed of different facets.26 Obviously, all the samples synthesized through this mixed-carbon-coating method are composed of homogeneous nano-particles. | ||
| Fig. 4 SEM images of samples D8-LiMnPO4 (a), D8-LiMn0.8Fe0.2PO4 (b), D8-LiMn0.5Fe0.5PO4 (c), D8-LiFePO4 (d). | ||
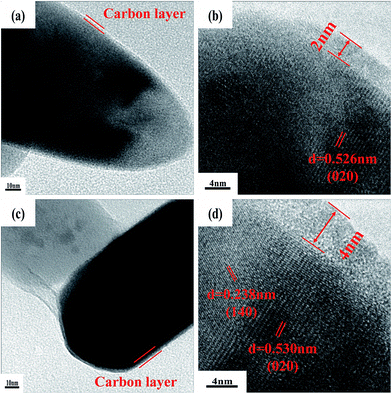
In order to study the influence of carbon content in LMFP samples, more careful investigations of LiMn0.5Fe0.5PO4 are performed. Fig. 5 shows the TEM and HRTEM images of D8- and D15-LiMn0.5Fe0.5PO4 samples. Apparently, a uniform carbon coating layer can be seen clearly on both samples (Fig. 5a and c). Nevertheless, the thickness of the carbon coating is about 2.0 and 4.0 nm for D8- (Fig. 5b) and D15-LiMn0.5Fe0.5PO4 (Fig. 5d), respectively. Such a uniform carbon layer should benefit from the use of ascorbic acid, as reported in our previous work.24 The N2-adsorption/desorption analysis and the pore size distributions of D8- and D15-LiMn0.5Fe0.5PO4 are also measured (Fig. S1†). Both samples give a type-IV isotherm from the N2-sorption analysis (Fig. S1a and b†), with a BET specific surface area of 33.5 and 22.1 m2 g−1, respectively. The pore size distributions (Fig. S1c and d†) show that the samples have bimodal pore-size distribution with a range of small mesoporous from 3 to 5 nm, which come from the pores within the LiMn0.5Fe0.5PO4 nano-particles, and another range of big mesopores from 16–100 nm, which are from the spaces between the nano-particles. However, D8-LiMn0.5Fe0.5PO4 shows much more small mesopores from 3 to 5 nm than D15-LiMn0.5Fe0.5PO4, suggesting some filling of carbon in the mesopores due to too thick carbon layer of D15-LiMn0.5Fe0.5PO4.
Table 1 lists the carbon contents of D8- and D15-LMFP samples measured by an infrared carbon-sulfur analyzer. Clearly, the carbon contents are about 2 wt% and 3.5 wt% for D8- and D15-LMFP samples, respectively. The results show that the amount of carbon residues is rather consistent regardless of the Fe content in LMFP. According to our knowledge on the industrial LiFePO4 products, about 2 wt% of carbon content is optimal for the electrochemical properties, meaning D8-LMFP samples are better than D15-LMFP, which is confirmed by our electrochemical measurements (see below).
| Glucose content (wt%) | Carbon content (wt%) | |||
|---|---|---|---|---|
| LiMnPO4 | LiMn0.8Fe0.2PO4 | LiMn0.5Fe0.5PO4 | LiFePO4 | |
| 8 | 2.02 | 1.84 | 2.11 | 2.00 |
| 15 | 3.55 | 3.17 | 3.87 | 3.21 |
3.3 Electrochemical properties of LMFP nanopowders
Fig. 6 shows the cyclic voltammograms (CV) of D8-LiMn1−xFexPO4 (x = 0, 0.2, 0.5) electrodes between 2.2 and 4.5 V and D8-LiFePO4 electrode between 2.2 and 4.0 V vs. Li/Li+. The separate redox peaks are well-defined and almost keep unchanged in all samples. As for the pure D8-LiFePO4 and D8-LiMnPO4 samples, a couple of redox peaks around 3.54/3.37 V and 4.33/3.90 V correspond to the transformation of LiFePO4 ⇆ FePO4, and LiMnPO4 ⇆ MnPO4, respectively. For the D8-LiMn0.5Fe0.5PO4 and D8-LiMn0.8Fe0.2PO4 samples, two couples of sharp redox peaks are measured and the ratio of the peak area corresponds to the stoichiometric ratio of the Mn/Fe. Generally, with the increased Mn/Fe ratio, the reaction between Mn2+ ⇆ Mn3+ becomes kinetically more difficult than the reaction Fe2+ ⇆ Fe3+, so that the voltage gap between the cathodic and anodic peaks of the Mn2+/Mn3+ usually becomes greater, being 0.15 V (x = 0.5), 0.25 V (x = 0.2), and 0.43 V (x = 0). The result proves that the iron substitution increases the lithium ion diffusion coefficient, and especially accelerates the transformation between Mn2+ ⇆ Mn3+.Fig. S2† and 7 give the galvanostatic cycling results of Li/LiMn1−xFexPO4@C (x = 0, 0.2, 0.5, 1) half-cells at room temperature (25 ± 2 °C). From Fig. S2,† it can be seen that the discharge specific capacity is 25.3 and 102.3 mA h g−1 for D15-LiMn0.8Fe0.2PO4 and D15-LiMn0.8Fe0.2PO4 at 20C. However, it is as high as 62.2 and 120.2 mA h g−1 at 20C for D8-LiMn0.8Fe0.2PO4 and D8-LiMn0.5Fe0.5PO4 (Fig. 7a). Obviously, a too thick carbon layer (3.5–4.0 nm) not only decreases the sample's surface area, but also seriously hinders the diffusion of lithium ions, leading to worse rate performance. In another case, if no glucose is added as the second carbon source, after the same calcination process, it can be seen that the primary particles of D0-LiFePO4 change from nanorods to nanoparticles with increased size of about 300–500 nm, some agglomerated large particles are also existed (Fig. S3†). The electrochemical performance is also very poor in Fig. S4.† Large polarization and very low capacity demonstrate the necessity of suitable carbon coating. The Li/D8-LMFP cells all show the excellent rate capability. For example, the specific discharge capacity of D8-LiMn0.8Fe0.2PO4 is 139.6, 137.4, 134.7, 126.2, 108.0, 92.8 and 62.2 mA h g−1 at 0.5, 1, 2, 5, 10, 15 and 20C discharge current density, which is rarely reported in the literature.27–36 Furthermore, D8-LiMn0.5Fe0.5PO4 and D8-LiFePO4 have not only high specific capacities (152.3 and 163.1 mA h g−1 at 0.1C, 120.2 and 140.2 mA h g−1 at 20C), but also very high average discharge voltage plateaus (3.733 and 3.415 V at 0.1C, 3.268 and 3.124 V at 20C) (Fig. 7b–c). The polarization evaluated by the charge and discharge plateaus is 32.3 mV for D8-LiFePO4 electrodes at 0.1C, which is smaller than most of literature data (Fig. S5a†).35–40
 | ||
| Fig. 7 Rate performance of D8-LMFP (0.5C charge) (a), the charge–discharge curves of D8-LiMn0.5Fe0.5PO4 (b) and D8-LiFePO4 (c), cycling performance of D8-LMFP at 1C (d). | ||
Fig. S5b† shows the initial discharge curves of D8-LiMn1−xFexPO4 (x = 0, 0.2, 0.5, 1) at 0.1C. The specific discharge capacity decreases with increasing Mn/Fe molar ratio, but a higher average discharge voltage plateau is clearly seen. Also, except for D8-LiMnPO4, other Fe-containing LMFP samples show very good cycling stability (Fig. 7d). At 1C rate, D8-LiMn1−xFexPO4 (x = 0, 0.2, 0.5, 1) can deliver a specific discharge capacity of 102.9, 134.7, 149.2 and 154.3 mA h g−1 at the 1st cycle, and retain 57.7% (59.4 mA h g−1), 95.2% (128.3 mA h g−1), 97.1% (145.1 mA h g−1) and 99.2% (153.0 mA h g−1) after 100 cycles, respectively. Obviously, D8-LiFePO4 demonstrates the best cycling and rate performance than other Mn-containing samples D8-LiMn1−xFexPO4 (x = 0, 0.2, 0.5). However, for practical applications, it can be seen in the Table 2, the energy density of LiMn0.5Fe0.5PO4 is the highest at 1C rate, even after 100 cycles. Taking into consideration of the energy density, cycling and rate performance, D8-LiMn0.5Fe0.5PO4 sample is more favourable in terms of energy density (vs. D8-LiFePO4) and rate property (vs. D8-LiMn0.8Fe0.2PO4).
| Electrochemical performance | D8-LiMn1−xFexPO4 | ||||
|---|---|---|---|---|---|
| x = 0 | x = 0.2 | x = 0.5 | x = 1 | ||
| Average discharge voltage (V) | 0.1C | 3.753 | 3.890 | 3.733 | 3.415 |
| 1C | 3.617 | 3.783 | 3.658 | 3.357 | |
| Energy density (W h kg−1) | 0.1C | 448 | 576 | 568 | 556 |
| 1C (1st) | 372 | 510 | 546 | 518 | |
| 1C (100th) | 204 | 476 | 523 | 513 | |
The EIS results (Fig. 8) are consistent with the sequence of rate performances of D8-LiMFP. All the Nyquist plots give similar spectra consisting of a depressed semicircle in the medium-frequency region and a straight line in the low-frequency region. The values of ohmic resistance (Rs) for all the cells are all at 1–2 ohm, but the charge transfer resistance (Rct) is 174, 142, 87 and 53 ohm for D8-LiMnPO4, D8-LiMn0.8Fe0.2PO4, D8-LiMn0.5Fe0.5PO4 and D8-LiFePO4, respectively, which demonstrates that the Fe substitution for Mn in D8-LiMn1−xFexPO4 can indeed accelerate the electron/ion transport.
 | ||
| Fig. 8 EIS of half cells Li/D8-LiMn1−xFexPO4 (x = 0, 0.2, 0.5, 1) after 3 cycles at 0.1C rate and the corresponding fitting using equivalent circuit (inset). | ||
The low temperature electrochemical performances of D8-LMFP are also desirable as shown in Fig. 9. For example, D8-LiMn0.5Fe0.5PO4 can deliver 109.4 mA h g−1 with an average discharge voltage as high as 3.476 V at 0.2C and −12 °C, while the sample D8-LiFePO4 shows more excellent low temperature performance with a capacity of 138.8 mA h g−1 and a voltage as high as 3.385 V (Fig. 9a). When the cells are cycled at −20 °C, the discharge capacity can achieve 94.1 and 123.8 mA h g−1 for D8-LiMn0.5Fe0.5PO4 and D8-LiFePO4 at 0.2C (Fig. 9b). Even at 3C rate, their specific capacities can still maintain at 71.7 and 82.3 mA h g−1, respectively. It is worth noting that the high average discharge voltages at −20 °C for D8-LiMn0.5Fe0.5PO4 (3.432 V, 0.2C) (Fig. 9c) and D8-LiFePO4 (3.190 V, 0.2C) (Fig. 9c) are rarely seen not only in published papers but also in commercial LIBs.41–43 To our knowledge, this is the first time to present a comparative study on the low temperature electrochemical performance of LiMn0.5Fe0.5PO4 and LiFePO4.
4. Conclusions
In summary, several mixed-carbon-coated LiMn1−xFexPO4 (x = 0, 0.2, 0.5, 1) powders with a uniform particle size distribution are synthesized through the novel solvothermal method. The obtained samples show optimal electrochemical performance when the carbon content is about 2 wt%. Both the tailored microstructures and uniform carbon coating layer are critical to achieve the excellent rate performance and low temperature electrochemical performance. The substitution of Fe2+ with Mn2+ can increase the discharge average voltage in some extent. However, with the increased Mn2+ in LMFP samples, it suffers from gradually declined electrochemical properties. The synthesized LiMn0.5Fe0.5PO4 sample has the optimal properties with a high energy density (546 W h kg−1), good cycleability (97.1%, 100 cycles) and rate capability (120.2 mA h g−1, 20C). It is a promising cathode material for high performance lithium-ion batteries.Acknowledgements
This study was supported by the National Science Foundation of China (grant no. 51577175), Hefei Center of Materials Science and Technology (2014FXZY006) and Education Ministry of Anhui Province (KJ2014ZD36). We are also grateful to Elementec Ltd in Suzhou.Notes and references
- A. K. Padhi, K. S. Nanjundaswamy and J. B. Goodenough, J. Electrochem. Soc., 1997, 144, 1188 CrossRef CAS.
- G. H. Li, H. Azuma and M. Tohda, Electrochem. Solid-State Lett., 2002, 5, A135 CrossRef CAS.
- K. Amine, H. Yasuda and M. Yamachi, Electrochem. Solid-State Lett., 2000, 3, 178 CrossRef CAS.
- J. Wolfenstine and J. Allen, J. Power Sources, 2004, 136, 150 CrossRef CAS.
- L. X. Yuan, Z. H. Wang, W. X. Zhang, X. L. Hu, J. T. Chen, Y. H. Huang and J. B. Goodenough, Energy Environ. Sci., 2011, 4, 269 CAS.
- V. Aravindan, J. Gnanaraj, Y. S. Lee and S. Madhavi, J. Mater. Chem. A, 2013, 1, 3518 CAS.
- L. F. Zhang, Q. T. Qu, L. Zhang, J. Li and H. H. Zheng, J. Mater. Chem. A, 2014, 2, 711 CAS.
- K. P. Wu, G. R. Hu, Z. D. Peng, Z. J. Zhang, Y. B. Cao and K. Du, RSC Adv., 2015, 5, 95020 RSC.
- J. Fan, Y. Yu, Y. Wang, Q. H. Wu, M. Zheng and Q. Dong, Electrochim. Acta, 2016, 194, 52 CrossRef CAS.
- K. Wang, Y. G. Wang, C. X. Wang and Y. Y. Xia, Electrochim. Acta, 2014, 146, 8 CrossRef CAS.
- K. P. Wu, G. R. Hu, Z. D. Peng, Y. B. Cao and K. Du, Electrochim. Acta, 2016, 196, 252 CrossRef CAS.
- H. Guo, C. Y. Wu, J. Xie, S. C. Zhang, G. S. Cao and X. B. Zhao, J. Mater. Chem. A, 2014, 2, 10581 CAS.
- X. N. Fu, Z. R. Chang, K. Chang, B. Li, H. W. Tang, E. Shangguan, X. Z. Yuan and H. J. Wang, Electrochim. Acta, 2015, 178, 420 CrossRef CAS.
- D. Y. Wang, C. Y. Ouyang, T. Drezen, I. Exnar, A. Kay, N. H. Kwon, P. Gouerec, J. H. Miners, M. K. Wang and M. Gratzel, J. Electrochem. Soc., 2010, 157, A225 CrossRef CAS.
- C. L. Hu, H. H. Yi, H. S. Fang, B. Yang, Y. C. Yao, W. H. Ma and Y. N. Dai, Electrochem. Commun., 2010, 12, 1784 CrossRef CAS.
- H. H. Yi, C. L. Hu, X. M. He and H. Y. Xu, Ionics, 2015, 21, 667 CrossRef CAS.
- L. L. Su, X. W. Li, H. Ming, J. Adkins, M. M. Liu, Q. Zhou and J. W. Zheng, J. Solid State Electrochem., 2014, 18, 755 CrossRef CAS.
- L. Wu, S. K. Zhong, J. J. Lu, J. Q. Liu and F. Lv, Ionics, 2013, 19, 1061 CrossRef CAS.
- T. Shiratsuchi, S. Okada, T. Doi and J. Yamaki, Electrochim. Acta, 2009, 54, 3145 CrossRef CAS.
- L. Q. Kou, F. J. Chen, F. Tao, Y. Dong and L. Chen, Electrochim. Acta, 2015, 173, 721 CrossRef CAS.
- P. F. Xiao, B. Ding, M. O. Lai and L. Lu, J. Electrochem. Soc., 2013, 160, A918 CrossRef CAS.
- K. Hoshina, T. Sasakawa, N. Takami, H. Munakata and K. Kanamura, J. Electrochem. Soc., 2015, 162, A2827 CrossRef CAS.
- Y. Hong, Z. L. Tang, Z. J. Hong and Z. T. Zhang, J. Power Sources, 2014, 248, 655 CrossRef CAS.
- B. K. Zou, H. Y. Wang, Z. Y. Qiang, Y. Shao, X. Sun, Z. Y. Wen and C. H. Chen, Electrochim. Acta, 2016, 196, 377 CrossRef CAS.
- K. Dokko, S. Koizumi, H. Nakano and K. Kanamura, J. Mater. Chem., 2007, 17, 4803 RSC.
- L. Wang, X. M. He, W. T. Sun, J. L. Wang, Y. D. Li and S. S. Fan, Nano Lett., 2012, 12, 5632 CrossRef CAS PubMed.
- L. T. Yang, Y. G. Xia, L. F. Qin, G. X. Yuan, B. Qiu, J. L. Shi and Z. P. Liu, J. Power Sources, 2016, 304, 293 CrossRef CAS.
- B. Z. Li, Y. Wang, L. Xue, X. P. Li and W. S. Li, J. Power Sources, 2013, 232, 12 CrossRef CAS.
- S. S. Li, Z. Su, A. Muslim, X. K. Jiang and X. Y. Wang, Ceram. Int., 2015, 41, 11132 CrossRef CAS.
- S. S. Li, Z. Su, X. K. Jiang and A. Muslim, Mater. Lett., 2015, 154, 29 CrossRef CAS.
- S. Y. Yan, C. Y. Wang, R. M. Gu, S. Sun and M. W. Li, J. Alloys Compd., 2015, 628, 471 CrossRef CAS.
- W. Xiang, E. H. Wang, M. Z. Chen, H. H. Shen, S. L. Chou, H. Chen, X. D. Guo, B. H. Zhong and X. L. Wang, Electrochim. Acta, 2015, 178, 353 CrossRef CAS.
- Z. X. Chi, W. Zhang, X. S. Wang, F. Q. Cheng, J. T. Chen, A. M. Cao and L. J. Wan, J. Mater. Chem. A, 2014, 2, 17359 CAS.
- X. Zhou, Y. Xie, Y. F. Deng, X. S. Qin and G. H. Chen, J. Mater. Chem. A, 2015, 3, 996 CAS.
- D. Xu, P. F. Wang and B. W. Shen, Ceram. Int., 2016, 42, 5331 CrossRef CAS.
- S. H. Ha and Y. J. Lee, Chem. –Eur. J., 2015, 21, 2132 CrossRef CAS PubMed.
- C. H. Zhang, L. Yao and Y. P. Qiu, J. Appl. Polym. Sci., 2016, 133, 43001 Search PubMed.
- J. L. Zhang, N. Nie, Y. Y. Liu, J. Wang, F. Yu, J. J. Gu and W. Li, ACS Appl. Mater. Interfaces, 2015, 7, 20134 CAS.
- J. Li, L. Zhang, L. F. Zhang, W. W. Hao, H. B. Wang, Q. T. Qu and H. H. Zheng, J. Power Sources, 2014, 249, 311 CrossRef CAS.
- M. Chen, C. Y. Du, B. Song, K. Xiong, G. P. Yin, P. J. Zuo and X. Q. Cheng, J. Power Sources, 2013, 223, 100 CrossRef CAS.
- G. L. Cai, R. S. Guo, L. Liu, Y. X. Yang, C. Zhang, C. Wu, W. N. Guo and H. Jiang, J. Power Sources, 2015, 288, 136 CrossRef CAS.
- C. L. Gong, Z. G. Xue, X. E. Wang, X. P. Zhou, X. L. Xie and Y. W. Mai, J. Power Sources, 2014, 246, 260 CrossRef CAS.
- Y. B. Lin, Y. Lin, T. Zhou, G. Y. Zhao, Y. D. Huang and Z. G. Huang, J. Power Sources, 2013, 226, 20 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12472k |
| This journal is © The Royal Society of Chemistry 2016 |