Controlled functionalization of graphene with carboxyl moieties toward multiple applications†
Zhongzheng Miao,
Xianglong Li* and
Linjie Zhi*
CAS Key Laboratory of Nanosystem and Hierarchical Fabrication, National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: lixl@nanoctr.cn; zhilj@nanoctr.cn
First published on 14th June 2016
Abstract
We here report a controlled functionalization strategy which enables the scalable production of a new kind of carboxyl-rich functionalized graphene without sacrificing the structural integrity and quality of the basal plane. The thus-resulting high-quality functionalized graphene is solution-processable and is capable of acting as a nearly intact two-dimensional scaffold for the uniform assembly of functional inorganic species, as an attractive electrode material for superior transparent conductive films, and also as the integral element of an interesting material system with continuously tunable hydrophilicity, all of which evidence their multifunctional potentials in different applications.
Graphene (G), a monolayer of carbon atoms densely packed into a two-dimensional honeycomb crystal lattice, has recently attracted tremendous interest for a broad spectrum of potential applications on account of its exceptional mechanical, electrical, thermal and optical properties.1–7 Toward macro applications, graphene oxide (GO),8–15 a well-known solution processable precursor of graphene, has been mostly studied as a universal starting material, which overcomes the processing difficulties of graphene due to its intrinsic insolubility. GO, which is generally obtained by solution oxidation of graphite with strong oxidizing reagents (e.g., potassium permanganate (KMnO4) and concentrated sulfuric acid (H2SO4) in a modified Hummers method) and can be further transformed into reduced graphene oxide (RGO) upon diverse reduction strategies,16–18 features excellent solution processing capability as well as massive manageability and manipulation properties, thanks to the presence of a large number of epoxy and hydroxyl groups on its basal plane and carboxyl groups (–COOH) on the peripheral interface.19,20 As a plus, the specific functional groups (e.g., –COOH) on the GO can be further applied as versatile anchoring points to direct the assembly of diverse species with matching functional groups (e.g., inorganic nanoparticles and polymers containing amino groups that can react with –COOH groups to form amides) on the GO, toward the construction of functional hybrid materials for diverse applications.8 With these merits, the glamorous role of GO has been widely demonstrated in different areas, representing a popular and facile way to utilize graphene materials on a large scale. Unfortunately, the GO, and also its reduced counterparts or derivatives, often bear numerous irremediable structural defects or large holes on the basal plane, which severely degrade the mechanical, thermal and electrical properties.21,22 This undoubtedly compromises the intrinsic potential of graphene and substantially limits its practical deployment.
Modifying the synthetic protocols of GO and/or employing new starting materials can reduce the possibility of forming impossible-to-heal defects or holes in the basal plane, being an effective way to chemically prepare high-quality GO. For example, sophisticated optimizations on oxidization conditions and post-treatment processes have led to the successful preparation of GO with significantly improved structural integrity as well as the selective formation of specific functional groups on the GO.23–25 Yet, in most cases the production yield and/or the layer-number controllability of GO need to be substantially improved, which is indeed not a trivial task. Hereof, it is highly desirable to develop new and efficient principles and methodologies to allow graphene with good dispersibility, scalable processibility, and specific functional moieties, whilst preserving its structural integrity.
Along with the mass production of high-quality graphene,26,27 the chemical functionalization28–34 (covalent and non-covalent) of graphene can be an alternative tactic to improve the dispersibility and processibility of graphene and endow the thus-functionalized graphene with specific functionalities, without perforating or even destroying graphene sheets. As an example, we recently developed a reversible chemical functionalization strategy which involves the controlled oxidation of graphene (specifically by using sodium chlorate (NaClO3) and H2SO4) to prepare functionalized graphene (HG) with highly removable oxygenated moieties (epoxy and hydroxyl groups) as well as its facile recovery toward nearly intact graphene (RHG) by mild hydrogen iodide (HI) reduction.35 Such reversible chemical functionalization strategy enables the facile and scalable processing of graphene and more importantly, guarantees the structural integrity and interfacial cleanliness of the graphene delivered into different macro materials and systems.
In this paper, we report a controlled functionalization strategy enabling the production of a new kind of solution-processable functionalized graphene with high structural integrity. The methodology is mainly adapted from the recipe for the HG, in which a certain amount of hydrogen peroxide (H2O2) is added to the reaction system of NaClO3 and H2SO4, instead of the use of KMnO4 and H2SO4 (Fig. 1). The resultant functionalized graphene (denoted as CG) is enriched with a large amount of carboxyl groups, and can be readily dispersed in water, thus allowing its facile manipulation and scalable processing; more importantly, the CG bears high structural integrity, a highly favorable feature for practical deployment. With these advantages, the CG acts as a nearly intact two-dimensional scaffold enabling the uniform assembly of functional nanoparticles or nanoparticle precursors (basic tin chloride (Sn(OH)Cl) in this work). Upon a mild chemical reduction, transparent conductive films (TCFs) made from reduced CG (RCG) exhibit superior performance compared to its counterpart with irreparable structural defects or larger holes. Furthermore, the CG, combined with HG, GO, and their reduced counterparts, constitutes a material system with continuously tunable hydrophilicity. These examples evidence the great potential of CG as a universal material platform for multifunctional applications.
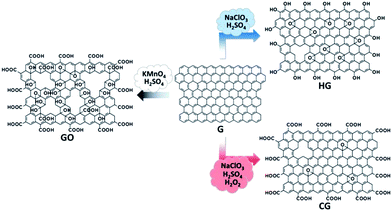 | ||
| Fig. 1 Schematic illustration of controlled functionalization of graphene (G) toward the synthesis of carboxyl-rich graphene (CG) and also control samples (HG, GO). | ||
The graphene used in this work was produced on a large scale by a modified interlayer catalytic exfoliation of graphite intercalation compounds (Fig. S1†).27 Upon the controlled chemical functionalization of graphene by using the oxidation system composed of NaClO3, H2SO4 and H2O2, the CG was facilely obtained (Fig. 2). As exhibited in Fig. 2a, the resultant CG can be readily dispersed in water, which is essentially important for the fabrication of macro materials and systems. The transmission electron microscopy (TEM) image shows that the CG bears a two-dimensional sheet-like modality (Fig. 2d); the selected area electron diffraction (SAED) image of CG further displays a six-fold symmetry and the presence of a single set of spots (Fig. 2e). This observation is consistent with that for the G (Fig. S1†), implying that the CG sheets retain a crystal structure even after experiencing the controlled chemical functionalization. The structural quality and also integrity is further depicted by Raman spectroscopy, a powerful tool to studying the structure of carbon materials. It is known that the Raman spectrum of GO involves a weak and obtuse 2D peak (Fig. S2†) due to the presence of large amounts of irreparable large structural defects/holes and also diverse oxygenated moieties. Towards the preservation of structural integrity, the HG obtained recently upon a controlled chemical oxidation processing still shows a similar picture mostly due to the existence of a large amount of hydroxyl and epoxy moieties on the basal planes.35 In stark contrast, a strong and sharp 2D peak is clearly observed in the Raman spectrum of CG (Fig. 3f), which strongly suggests the preservation of the sp2 carbon basal structures after the controlled chemical functionalization,36,37 consistent with the TEM observations (Fig. 2 and S3†). Furthermore, it should be mentioned that the thickness of individual CG sheets is generally less than 1 nm as shown in Fig. 2c.
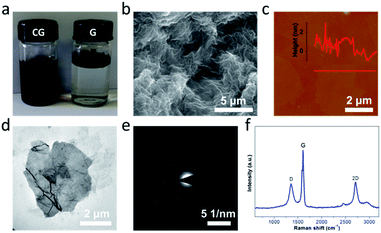 | ||
| Fig. 2 Structural characterization of CG. (a) Photographs of CG and G in water. (b) SEM image, (c) AFM image, (d) TEM image, (e) SAED pattern, and (f) Raman spectrum of CG. | ||
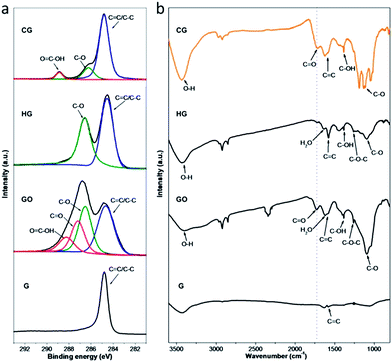 | ||
| Fig. 3 Characterization of functional moieties of CG and also control samples (HG, GO and G). (a) C1s XPS spectra. (b) FTIR spectra. | ||
X-ray photoelectron spectroscopy (XPS) analyses were performed to further unravel the functional moieties on the CG and also control samples. As exhibited in Fig. 3a, all the C1s XPS spectra show a peak at 284.7 eV, which can be attributed to the non-oxygenated carbons in aromatic rings (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–C). Besides, the deconvoluted C1s XPS spectra of both HG and GO display a quite strong peak assignable to C–O, reflecting that the hydroxyl and epoxy groups are the major oxygenated moieties on these sheets as schematically illustrated in Fig. 1. Therewith, two additional peaks with weaker intensity at higher binding energies are observed in the C1s spectrum of GO, which can be assigned to the carbonyl carbon (C
C/C–C). Besides, the deconvoluted C1s XPS spectra of both HG and GO display a quite strong peak assignable to C–O, reflecting that the hydroxyl and epoxy groups are the major oxygenated moieties on these sheets as schematically illustrated in Fig. 1. Therewith, two additional peaks with weaker intensity at higher binding energies are observed in the C1s spectrum of GO, which can be assigned to the carbonyl carbon (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and the carboxyl carbon (O
O) and the carboxyl carbon (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH) existing mostly at the edges of GO sheets, consistent with the diverse nature of oxygenated moieties on the GO sheets derived from graphite as reported elsewhere.38 In comparison, the deconvoluted C1s XPS spectrum of CG shows a C–O peak yet with significantly weakened intensity, implying that the hydroxyl and epoxy groups on the basal planes of the CG sheets are limited; besides, distinctly different from that of the HG, the C1s spectrum of CG displays an intense peak corresponding to the carboxyl carbon. These observations indicate the introduction of specific oxygenated moieties on the CG sheets via the controlled functionalization developed here, mostly, carboxyl groups residing at the edges of the CG sheets (Fig. 1), since their high structural quality as disclosed by the above Raman and TEM analyses. It should be noted that the oxygen content in the CG is estimated to be around 18% (Table S1†), which reveals the introduction of a high amount of carboxyl moieties as the majority onto the CG sheets, providing an explanation for their good dispersibility in water. The difference in oxygenated moieties on these samples has been further evidenced by their Fourier transform infrared spectroscopy (FTIR) spectra (Fig. 3b). Compared with that of G, the FTIR spectrum of HG indicates the presence of C–O (1090 cm−1), C–O–C (1258 cm−1), and C–OH (1385 cm−1), in agreement with the existence of hydroxyl and epoxy groups on the HG.35 Different from that scenario, the FTIR spectrum of CG shows the presence of C
C–OH) existing mostly at the edges of GO sheets, consistent with the diverse nature of oxygenated moieties on the GO sheets derived from graphite as reported elsewhere.38 In comparison, the deconvoluted C1s XPS spectrum of CG shows a C–O peak yet with significantly weakened intensity, implying that the hydroxyl and epoxy groups on the basal planes of the CG sheets are limited; besides, distinctly different from that of the HG, the C1s spectrum of CG displays an intense peak corresponding to the carboxyl carbon. These observations indicate the introduction of specific oxygenated moieties on the CG sheets via the controlled functionalization developed here, mostly, carboxyl groups residing at the edges of the CG sheets (Fig. 1), since their high structural quality as disclosed by the above Raman and TEM analyses. It should be noted that the oxygen content in the CG is estimated to be around 18% (Table S1†), which reveals the introduction of a high amount of carboxyl moieties as the majority onto the CG sheets, providing an explanation for their good dispersibility in water. The difference in oxygenated moieties on these samples has been further evidenced by their Fourier transform infrared spectroscopy (FTIR) spectra (Fig. 3b). Compared with that of G, the FTIR spectrum of HG indicates the presence of C–O (1090 cm−1), C–O–C (1258 cm−1), and C–OH (1385 cm−1), in agreement with the existence of hydroxyl and epoxy groups on the HG.35 Different from that scenario, the FTIR spectrum of CG shows the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O containing species on the sheets, similar to that of GO. It is noteworthy that, yet, the relative intensity ratio of the C
O containing species on the sheets, similar to that of GO. It is noteworthy that, yet, the relative intensity ratio of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C–O peaks for the spectrum of CG is much higher than that for GO, indicating the high content of C
O/C–O peaks for the spectrum of CG is much higher than that for GO, indicating the high content of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O containing species in the CG. Combined with the above XPS results, this observation evidently verifies the enrichment of carboxyl moieties on the CG sheets via our controlled chemical functionalization.
O containing species in the CG. Combined with the above XPS results, this observation evidently verifies the enrichment of carboxyl moieties on the CG sheets via our controlled chemical functionalization.
The assembly of a series of nanoparticles and/or nanoparticle precursors on graphene has resulted in the construction of diverse functional hybrid materials for different applications.8,39 One critical factor dominating the performance of these hybrid materials is to control the size and assembly uniformity of functional nanoparticles. As a proof-of-concept demonstration, the assembly of Sn(OH)Cl nanoparticles on the CG and also control samples was carried out in this work. In the case of GO (Fig. 4e and f), small Sn(OH)Cl nanoparticles, mingled with their large aggregates with an average size of around 100 nm, are observed on the basal planes of GO. It can be assumed that these large Sn(OH)Cl aggregates identify the locations of the large structural defects or holes on the basal planes of GO, since the edges of these holes are decorated with numerous carbonyl and/or carboxyl groups which greatly abduct the accumulation of nanoparticle precursors (for example, Sn2+) and consequent propagation of large Sn(OH)Cl aggregates. A similar phenomenon has also been reported, in which the rings or aggregates of crystalline platinum nanoparticles are located around the large holes of GO.40 In stark contrast, as shown in Fig. 4a–d, the uniform Sn(OH)Cl nanoparticles with sizes less than 10 nm are homogeneously distributed on the CG and also HG sheets, demonstrating that the CG and also HG can be used as a nearly intact two-dimensional skeleton or template for the uniform assembly of other inorganic nanostructured materials. That is, this observation also evidently illustrates the structural integrity of these CG/HG sheets after experiencing controlled chemical oxidizing processes.
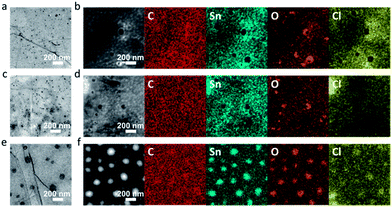 | ||
| Fig. 4 Characterization of Sn(OH)Cl nanoparticles assembled onto CG (a, b), HG (c, d), and GO (e, f). (a, c, e) TEM images. (b, d, f) Elemental mapping images. | ||
To demonstrate the potential of CG in the field of transparent conductive films, the water-dispersible CG was processed into two-dimensional macro films on poly(ethylene terephthalate) (PET) substrates by our previously-developed rod-coating method.41 Upon a mild room-temperature HI reduction, the RCG TCFs were facilely obtained. As shown in Fig. 5a, the sheet resistance of RCG TCFs is constantly substantially lower than that of RGO-based TCFs at the same transmittance. As an example, with a transmittance of ∼80% at 550 nm, the RGO TCF has a sheet resistance of ∼18 kΩ sq−1, a typical value for TCFs made from similar RGO materials.35 In comparison, the RCG TCF exhibits a sheet resistance of ∼8 kΩ sq−1 at 80% transparency, which is lower than half that of the RGO TCF. Since the same reduction process, we believe that the higher electrical conductivity of RCG TCFs is mostly related to the high structural integrity of CG and consequent RCG sheets. As the slight difference in sheet resistances between RCG TCFs and RHG TCFs can be attributed to an interface effect induced by the remaining carboxyl groups on the peripheral interface of the CG sheets as confirmed by the XPS data (Fig. 5b), the similar electrical conductivity between RCG TCFs and RHG TCFs rather depicts the important role of preserving the structural integrity of individual graphene sheets. Furthermore, it can be speculated that the remaining carboxyl moieties on the RCG may function as interesting interfacing points to enable specific molecular sensing and bio-recognition based on RCG TCFs.
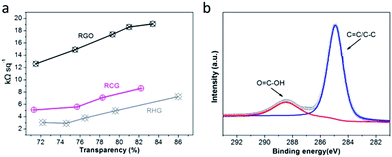 | ||
| Fig. 5 Characterization of TCFs made from RCG and control samples. (a) Sheet resistance and transmittance at 550 nm of TCFs. (b) C1s XPS spectrum of RCG. | ||
Last but not least, the hydrophilic properties of the CG and also control samples were investigated. As shown in Fig. 6a, the GO film bears a water contact angle of 15.4°, attributable to the presence of a large amount of diverse oxygenated moieties. In comparison, the CG and HG films show higher water contact angles (58.5° and 42.1°, respectively), which mainly originate from the difference in the amount of hydrophilic oxygenated moieties in both materials (Table S1†). It should be noted that the observed difference in water contact angles of the CG and HG films can be associated with a low degree of functionalization of the hydrophobic basal planes of the CG sheets, in comparison with their high degree of edge functionalization with hydrophilic carboxyl groups. After HI reduction, the contact angle of water upon the RCG film was measured to be 77.8°, which is slightly lower than that of the RHG film (84.4°). Given the similar structural integrity of RCG and RHG sheets, the difference observed can be attributed to the presence of carboxyl groups remaining in the RCG film which really benefit the contact with water. As shown in Fig. 6b, the CG, combined with HG, GO, and their reduced ones, truly forms a graphene-based material system with continuously tunable hydrophilicity, which would be highly desirable for the fields especially requiring specific surface design and engineering.42
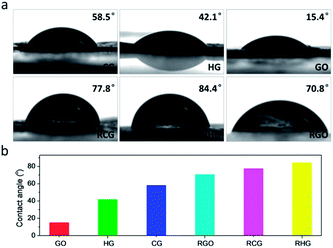 | ||
| Fig. 6 Contact angle measurements of CG. (a) A water droplet on a series of films made from CG and control samples as annotated. (b) Summary of contact angles. | ||
Conclusions
In conclusion, a controlled functionalization strategy has been developed for facilely modifying graphene. The methodology, adapted from the recipe of hydroxyl and epoxy-functionalized graphene, enriches the thus-resulted functionalized graphene with a large amount of carboxyl groups on the peripheral interface, without sacrificing the structural integrity of the basal plane. The thus-resulted solution-processable and high-quality carboxyl-rich functionalized graphene can act as the nearly intact two-dimensional scaffold for the uniform assembly of functional inorganic species, as the charming electrode material for transparent conductive films, and as the integral element of a material system with continuously tunable hydrophilicity. In general, this study provides a unique and universal graphene material platform for different areas especially requiring the high structural integrity and also intrinsic properties of graphene, and would expand the potential of graphene in a series of macro applications including energy storage and conversion, sensing, and so forth.Acknowledgements
The authors acknowledge support from the National Natural Science Foundation of China (Grant No. 51302045, 51425302), the Ministry of Science and Technology of China (2012CB933403), and the Chinese Academy of Sciences.References
- F. Bonaccorso, L. Colombo, G. Yu, M. Stoller, V. Tozzini, A. C. Ferrari, R. S. Ruoff and V. Pellegrini, Science, 2015, 347, 1246501 CrossRef PubMed.
- R. Raccichini, A. Varzi, S. Passerini and B. Scrosati, Nat. Mater., 2015, 14, 271–279 CrossRef CAS PubMed.
- L. Liao, H. Peng and Z. Liu, J. Am. Chem. Soc., 2014, 136, 12194–12200 CrossRef CAS PubMed.
- W. Ren and H.-M. Cheng, Nat. Nanotechnol., 2014, 9, 726–730 CrossRef CAS PubMed.
- K. Kostarelos and K. S. Novoselov, Science, 2014, 344, 261–263 CrossRef CAS PubMed.
- D. Wei, B. Wu, Y. Guo, G. Yu and Y. Liu, Acc. Chem. Res., 2013, 46, 106–115 CrossRef CAS PubMed.
- K. S. Novoselov, V. I. Fal'ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, Nature, 2012, 490, 192–200 CrossRef CAS PubMed.
- Z. Li, S. Wu, W. Lv, J. Shao, F. Kang and Q.-H. Yang, Small, 2016, 12, 2674–2688 CrossRef CAS PubMed.
- H. Cheng, M. Ye, F. Zhao, C. Hu, Y. Zhao, Y. Liang, N. Chen, S. Chen, L. Jiang and L. Qu, Adv. Mater., 2016, 28, 3305–3312 CrossRef CAS PubMed.
- L. Peng, Z. Xu, Z. Liu, Y. Wei, H. Sun, Z. Li, X. Zhao and C. Gao, Nat. Commun., 2015, 6, 5716 CrossRef CAS PubMed.
- S. Han, D. Wu, S. Li, F. Zhang and X. Feng, Adv. Mater., 2014, 26, 849–864 CrossRef CAS PubMed.
- C. Li and G. Shi, Adv. Mater., 2014, 26, 3992–4012 CrossRef CAS PubMed.
- D. R. Dreyer, A. D. Todd and C. W. Bielawski, Chem. Soc. Rev., 2014, 43, 5288–5301 RSC.
- J. Luo, J. Kim and J. Huang, Acc. Chem. Res., 2013, 46, 2225–2234 CrossRef CAS PubMed.
- D. Li, M. B. Mueller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- S. Mao, H. Pu and J. Chen, RSC Adv., 2012, 2, 2643–2662 RSC.
- C. Chen, Q. Zhang, M. Yang, C.-H. Huang, Y. Yang and M. Wang, Carbon, 2012, 50, 3572–3584 CrossRef CAS.
- W. M. A. El Rouby, RSC Adv., 2015, 5, 66767–66796 RSC.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- J. Kim, L. J. Cote, F. Kim, W. Yuan, K. R. Shull and J. Huang, J. Am. Chem. Soc., 2010, 132, 8180–8186 CrossRef CAS PubMed.
- S. Eigler and A. Hirsch, Angew. Chem., Int. Ed., 2014, 53, 7720–7738 CrossRef CAS PubMed.
- K. Erickson, R. Erni, Z. Lee, N. Alem, W. Gannett and A. Zettl, Adv. Mater., 2010, 22, 4467–4472 CrossRef CAS PubMed.
- J. Chen, Y. Zhang, M. Zhang, B. Yao, Y. Li, L. Huang, C. Li and G. Shi, Chem. Sci., 2016, 7, 1874–1881 RSC.
- S. Eigler, M. Enzelberger-Heim, S. Grimm, P. Hofmann, W. Kroener, A. Geworski, C. Dotzer, M. Roeckert, J. Xiao, C. Papp, O. Lytken, H.-P. Steinrueck, P. Mueller and A. Hirsch, Adv. Mater., 2013, 25, 3583–3587 CrossRef CAS PubMed.
- I.-Y. Jeon, Y.-R. Shin, G.-J. Sohn, H.-J. Choi, S.-Y. Bae, J. Mahmood, S.-M. Jung, J.-M. Seo, M.-J. Kim, D. W. Chang, L. Dai and J.-B. Baek, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 5588–5593 CrossRef CAS PubMed.
- K. R. Paton, E. Varrla, C. Backes, R. J. Smith, U. Khan, A. O'Neill, C. Boland, M. lotya, O. M. Istrate, P. King, T. Higgins, S. Barwich, P. May, P. Puczkarski, I. Ahmed, M. Moebius, H. Pettersson, E. Long, J. Coelho, S. E. O'Brien, E. K. McGuire, B. M. Sanchez, G. S. Duesberg, N. McEvoy, T. J. Pennycook, C. Downing, A. Crossley, V. Nicolosi and J. N. Coleman, Nat. Mater., 2014, 13, 624–630 CrossRef CAS PubMed.
- X. Geng, Y. Guo, D. Li, W. Li, C. Zhu, X. Wei, M. Chen, S. Gao, S. Qiu, Y. Gong, L. Wu, M. Long, M. Sun, G. Pan and L. Liu, Sci. Rep., 2013, 3, 464–467 Search PubMed.
- K. S. Mali, J. Greenwood, J. Adisoejoso, R. Phillipson and S. D. Feyter, Nanoscale, 2015, 7, 1566–1585 RSC.
- A. Criado, M. Melchionna, S. Marchesan and M. Prato, Angew. Chem., Int. Ed., 2015, 54, 10734–10750 CrossRef CAS PubMed.
- L. Dai, Acc. Chem. Res., 2013, 46, 31–42 CrossRef CAS PubMed.
- C. K. Chua and M. Pumera, Chem. Rev., 2013, 42, 3222–3233 RSC.
- J. Malig, N. Jux and D. M. Guldi, Acc. Chem. Res., 2013, 46, 53–64 CrossRef CAS PubMed.
- A. Hirsch, J. M. Englert and F. Hauke, Acc. Chem. Res., 2013, 46, 87–96 CrossRef CAS PubMed.
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Chem. Rev., 2012, 112, 6156–6214 CrossRef CAS PubMed.
- Z. Miao, X. Li, X. Zhang, M. Zhou, J. Ning, L. Miao, X. Qiu, M. Jin and L. Zhi, Adv. Mater. Interfaces, 2016, 3, 1500842 CrossRef.
- S. Pei, J. Zhao, J. Du, W. Ren and H. Cheng, Carbon, 2010, 48, 4466–4474 CrossRef CAS.
- M. S. Dresselhaus, A. Jorio, M. Hofmann, G. Dresselhaus and R. Saito, Nano Lett., 2010, 10, 751–758 CrossRef CAS PubMed.
- C. Mattevi, G. Eda, S. Agnoli, S. Miller, K. A. Mkhoyan, O. Celik, D. Mastrogiovanni, G. Granozzi, E. Garfunkel and M. Chhowalla, Adv. Funct. Mater., 2009, 19, 2577–2583 CrossRef CAS.
- X. Huang, X. Qi, F. Boey and H. Zhuang, Chem. Soc. Rev., 2012, 41, 666–686 RSC.
- X. Zhao, C. M. Hayner, M. C. Kung and H. H. Kung, ACS Nano, 2011, 5, 8739–8749 CrossRef CAS PubMed.
- J. Wang, M. Liang, Y. Fang, T. Qiu, J. Zhang and L. Zhi, Adv. Mater., 2012, 24, 2874–2878 CrossRef CAS PubMed.
- H. Chang, J. Qin, P. Xiao, Y. Yang, T. Zhang, Y. Ma, Y. Huang and Y. Chen, Adv. Mater., 2016, 28, 3504–3509 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12470d |
| This journal is © The Royal Society of Chemistry 2016 |
