Facile controllable hydrothermal route for a porous CoMn2O4 nanostructure: synthesis, characterization, and textile dye removal from aqueous media
Abstract
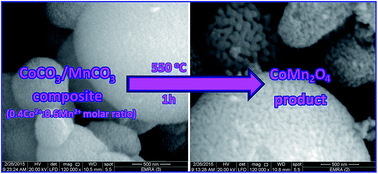
We herein report the synthesis of a pure sphere-like spinel CoMn2O4 nanostructure using a facile and surfactant-free hydrothermal approach followed by a thermal decomposition of the as-prepared CoCO3/MnCO3 composite precursor. Various factors affecting the hydrothermal reaction of cobalt chloride, manganese chloride, and ammonium hydrogen carbonate have been investigated to synthesize a pure CoCO3/MnCO3 composite precursor. Calcination of the CoCO3/MnCO3 composite (synthesized using 0.4Co2+ : 0.6Mn2+ molar ratio) at 550 °C for 1 h gave the pure sphere-like spinel CoMn2O4 nanostructure product (∼16 nm), but the other carbonate composites (synthesized using other molar ratios) did not generate pure spinel CoMn2O4 on calcination. The as-prepared products were identified employing XRD, FT-IR, TEM, EDS, FE-SEM, zeta potential, TG, and BET analyses. The as-prepared spinel CoMn2O4 product showed high adsorption capacity (132 mg g−1) for the removal of Reactive Black 5 (RB5) dye from aqueous media. The pseudo-second-order kinetics and Langmuir isotherm models described well the experimental adsorption results. The adsorption of RB5 dye on the as-prepared adsorbent is an endothermic, spontaneous, and physisorption process according to the calculated thermodynamic constants: ΔH0 (22.144 kJ mol−1), ΔG0 (from −4.321 to −6.990 kJ mol−1), and Ea (20.916 kJ mol−1), respectively. The as-prepared spinel CoMn2O4 adsorbent showed high stability, reusability, and high adsorption capacity implying its efficiency in removing the RB5 textile dye from aqueous media.

 Please wait while we load your content...
Please wait while we load your content...