Roles of oxygen and nitrogen in control of nonlinear resistive behaviors via filamentary and homogeneous switching in an oxynitride thin film memristor†
Abstract
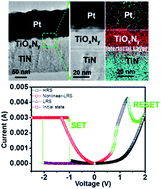
A TiOxNy thin film, which contains controllable concentrations of oxygen and nitrogen by a single-step reactive sputtering process using a non-symmetric Pt electrode as top electrode and TiN as bottom electrode, exhibiting non-linear I–V behavior, was proposed and demonstrated. A switching model of the non-linear I–V switching was built based on diffusion of oxygen vacancies in the TiOxNy film with different ratios of O and N after the SET process. Effects on the switching relationship between TiOxNy and electrodes were investigated to optimize the best conditions for the non-linear behavior. The origin of the nonlinear property was investigated in detail by changing the compositions of oxygen and nitrogen in the TiOxNy thin film. We believe that these findings would open up opportunities to exploit resistive switching mechanisms and simple memristor stacking in next generation crossbar array applications.

 Please wait while we load your content...
Please wait while we load your content...