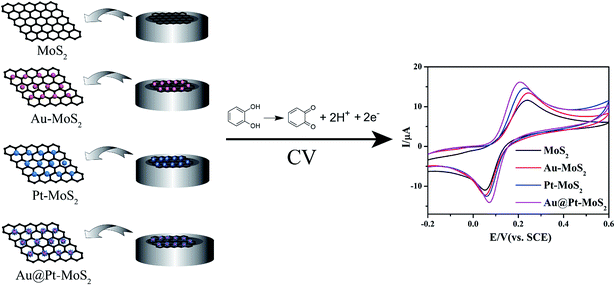
Improving performance of MoS2-based electrochemical sensors by decorating noble metallic nanoparticles on the surface of MoS2 nanosheet†
Shao Sua,
Wenfang Caoa,
Chi Zhanga,
Xiaoyan Hana,
Huan Yua,
Dan Zhua,
Jie Chaoa,
Chunhai Fanab and
Lianhui Wang*a
aKey Laboratory for Organic Electronics & Information Displays (KLOEID), Institute of Advanced Materials (IAM), Jiangsu National Syngerstic Innovation Center for Advanced Materials (SICAM), Nanjing University of Posts & Telecommunications, 9 Wenyuan Road, Nanjing 210023, China. E-mail: iamlhwang@njupt.edu.cn
bDivision of Physical Biology, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201800, China
First published on 8th August 2016
Abstract
Recently, the use of a molybdenum disulfide (MoS2) nanosheet as a sensing platform has attracted more and more scientists' attention due to its graphene-like structure and excellent physical/chemical properties. Herein, the performances of MoS2-based electrochemical sensors were improved by decorating noble metallic nanoparticles on the surface of the MoS2 nanosheet. Taking catechol (CC) as an analyte, it could be found that the MoS2 nanosheet and its derivatives have excellent electrocatalytic abilities toward CC. More interestingly, the gold nanoparticles-decorated MoS2 nanosheet (Au–MoS2), platinum nanoparticles-decorated MoS2 nanosheet (Pt–MoS2) and gold–platinum core–shell nanoparticles-decorated MoS2 nanosheet (Au@Pt–MoS2) modified electrodes exhibited better detection performances than the pure MoS2 nanosheet and metallic nanoparticles modified electrodes due to their synergistic effect. As expected, the Au@Pt–MoS2 nanocomposites modified electrode exhibited the linear range of 2–1000 μM and the detection limit of 0.44 μM for CC detection, which was better than all MoS2-based nanomaterials. All the MoS2-based electrochemical sensing platforms were employed to determine CC both in buffer and in real samples with satisfactory results, suggesting MoS2-based nanomaterials maybe the potential candidates for constructing electrochemical sensors for chemical and biological molecules detection.
1. Introduction
Molybdenum disulfide (MoS2) is a member of the layered transition-metal dichalcogenide family, which easily exfoliated to a few layers or even to a single layer because of the weak van der Waals interactions.1–4 As a graphene-like layered nanomaterial, MoS2 has many properties similar to graphene, such as abundant storage, good mechanical strength, large surface area and easy functionalization.5 More importantly, the thickness of a MoS2 nanosheet greatly influences its indirect-to-direct bandgap transition (from 1.2 to 1.9 eV),6,7 which makes MoS2 a charming material for applications in photoluminescence,8 transistors,9 catalysts for hydrogen evolution,10 sensors,11–14 lithium batteries15 and so on. Among them, constructing MoS2-based sensors combined with fluorescence, electrochemistry and surface-enhanced Raman scattering techniques for target molecules detection has gradually attracted more and more scientists' attention.1 For example, Zhu et al. utilized the fluorescence quenching ability of MoS2 to construct MoS2-based biosensors for homogeneous detection of biomolecules.14 Li's group fabricated an electrochemical biosensor for DNA and RNA detection based on thionin–MoS2 nanocomposite.16As we know, nanohybrids always exhibit obvious synergistic effects, which possess better performance than single component. Therefore, MoS2-based hybrid nanomaterials have been developed, such as MoS2–graphene,17 MoS2–organic compounds18 and MoS2–noble metallic nanoparticles.19 Among these MoS2-based nanohybrids, noble metallic nanoparticles-decorated MoS2 nanohybrids received an increasing interest by researchers. Zhang's and Wang's groups systematically investigated four noble metallic nanoparticles decorated on the surface of MoS2 nanosheets via different synthesized methods, such as Au, Ag, Pt and Pd nanoparticles. Moreover, they found such noble metallic nanoparticles–MoS2 nanocomposites showed higher catalytic activity compared with commercial Pt catalysts.20,21 Recently, Su et al. had successfully synthesized Au–Pt core–shell bimetallic nanoparticles-decorated MoS2 nanocomposites (Au@Pt–MoS2), which exhibited better catalytic activity toward methanol oxidation than that of Au–MoS2 and Pt–MoS2 nanocomposites.22 On the basis of successful synthesis, noble metallic nanoparticles-decorated MoS2 nanocomposites have been extensively used to construct sensors for chemical and biological molecules detection. Kuru et al. used Pd–MoS2 nanocomposites as a sensing platform for hydrogen detection.23 Wang's and Sow's groups employed Au–MoS2 nanocomposites as a surface-enhanced Raman scattering (SERS)-active substrate, which was used to high sensitive detection of Rhodamine 6G and methylene blue.24,25 Our group also used Au–MoS2 nanocomposites as electrochemical sensing platforms for neurotransmitters, H2O2, small biological molecules and protein detection.12,26–29
Inspired by these exciting studies, it prompts us to explore the performances of MoS2 nanosheet and its derivatives. Herein, we choose catechol (CC) as an analyte to study the performance of four MoS2-based electrochemical sensing platforms, including MoS2 nanosheet, gold nanoparticles-decorated MoS2 nanocomposites (Au–MoS2), platinum nanoparticles-decorated MoS2 nanocomposites (Pt–MoS2) and gold–platinum core–shell bimetallic nanoparticles-decorated MoS2 nanocomposites (Au@Pt–MoS2). CC is a typical heavy environmental pollutant due to its high toxicity and low degradability in the ecological environment. It can causes eczematous dermatitis, depression of the central nervous system (CNS) and a prolonged rise of blood pressure with the increasing doses of CC.30,31 Therefore, accurate, sensitive, fast and low-cost detection of CC is very meaningful to environmental monitoring and human health. As shown in Fig. 1, all four MoS2-based modified electrodes exhibit excellent electrocatalytic ability toward CC. It can be found that CC shows a pair of more well-defined and reversible redox peaks at Au@Pt–MoS2 film modified electrode than that at other MoS2-based modified electrodes. Moreover, Au@Pt–MoS2-based electrochemical sensor exhibits the widest linear range (2–1000 μM) and the lowest detection limit (0.44 μM) for CC detection in four MoS2-based sensors under the same condition. These results suggest that Au@Pt–MoS2 nanocomposite has better electrocatalytic performance than other MoS2-based nanocomposites. All MoS2-based electrochemical sensors are successfully employed to determine CC in real water sample with satisfactory recovery, indicating those MoS2-based nanomaterials maybe potential sensing platform candidates for chemical or biological molecules detection.
2. Experimental section
2.1. Apparatus
Cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and differential pulse voltammetry (DPV) were performed on an Autolab PGSTAT302 (Metrohm China Ltd, Switzerland). A conventional three-electrode system was used in this experiment. Different modified glassy carbon electrodes (GCE) (3.0 mm in diameter) was used as working electrode; a platinum wire and a saturated calomel electrode (SCE) were used as the auxiliary electrode and the reference electrode, respectively. The transmission electron microscopy (TEM) was employed to characterize MoS2 nanosheet and MoS2-based nanocomposites (Philips CM 200). The thickness of MoS2 nanosheet was examined with atomic force microscope (AFM, Bruker).2.2. Reagents and chemicals
Potassium hexacyanoferrate(III) (K3Fe(CN)6, ≥99.5%), potassium hexacyanoferrate(II) trihydrate (K4Fe(CN)6·3H2O, ≥99.5%), disodium hydrogen phosphate (Na2HPO4, ≥99.0%), sodium dihydrogen phosphate dehydrate (NaH2PO4·2H2O, ≥99.0%), catechol (CC, ≥98.0%) and ascorbic acid (AA, ≥99.7%) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Gold(III) tetrachloride trihydrate (HAuCl4·3H2O, ≥47.8%), sodium citrate tribasic dehydrate (Na3C6H5O7·2H2O, ≥99.5%), potassium tetrachloroplatinate(II) (K2PtCl4, 99.99%) and hexadecyltrimethylammonium bromide (CTAB, 96%) were purchased from Sigma (USA). Polyvinyl pyrrolidone (PVP) was purchased from Alfa Aesar (Tianjin, China). Carboxymethyl cellulose sodium (CMC) and sodium borohydride (NaBH4, 98%) were purchased from Aladdin Industrial Corporation (Shanghai, China). All chemicals were directly used without further purification. All solutions were prepared with Milli-Q water from a Milli-pore system.2.3. Synthesis of different MoS2-based nanomaterials
MoS2 nanosheet was prepared according to our previous works.25,29 The exfoliated MoS2 nanosheet was stored at 4 °C for further application. Au–MoS2, Pt–MoS2 and Au@Pt–MoS2 nanocomposites were synthesized by microwave-assisted hydrothermal method and oil-bath method. For Au–MoS2 synthesis, 100 μL PVP (5%) was added into 4 mL MoS2 (0.025 mg mL−1) solution and mixed for several minutes. Then, 200 μL HAuCl4 (10 mM) was injected into the solution. After this, the reaction mixture was heated to 60 °C for 5 minutes in the microwave reactor. Finally, the product of Au–MoS2 was purified at least twice by centrifugation. It should be noted that the Au–MoS2 nanoseed synthesized using the same method. Gold nanoparticle in Au–MoS2 nanoseed (100 μL HAuCl4) is smaller than that in Au–MoS2 nanocomposite (200 μL HAuCl4) due to the fewer volume of HAuCl4 (Fig. S1†). For Pt–MoS2 synthesis, 100 μL (50 mM) CMC, 50 μL (100 mM) NaBH4 and 300 μL (5 mM) H2PtCl6 were added into 10 mL (10 μg mL−1) MoS2 nanosheets solution. Then the reaction mixture was heated to 100 °C for 5 minutes in the microwave reactor. Finally, the product of Pt–MoS2 nanocomposite was centrifuged at least twice for the purification. Au@Pt–MoS2 nanocomposite was synthesized according to our previous work.22 0.5 mL 10 mM CTAB, 1 mL 100 mM AA and 100 μL 5 mM H2PtCl6 was added into 2 mL Au–MoS2 seed solution. The mixture was then heated on a hotplate at approximately 100 °C for 6 min to form Au@Pt–MoS2 nanocomposite. Finally, all the composites were stored at 4 °C for future use.2.4. Preparation of different MoS2-based modified electrodes
The GCE was firstly polished with 0.3 and 0.05 mm alumina slurry, respectively. After cleaning by ethanol and ultrapure water for several minutes, the GCE was dried under nitrogen stream. Then, 5 μL as-prepared MoS2 nanosheet was dropped onto the cleaned electrode to form MoS2 film modified electrode, which defined as MoS2/GCE. Similarly, Au/GCE, Au–MoS2/GCE, Pt/GCE, Pt–MoS2/GCE, Au@Pt/GCE and Au@Pt–MoS2/GCE were prepared by using the same procedure.3. Results and discussion
3.1. Characterization of MoS2-based nanomaterials
TEM was used to characterize the morphologies of MoS2 nanosheet and noble metallic nanoparticles-decorated MoS2 nanocomposites. As shown in Fig. 2A, MoS2 nanosheet possessed a typical graphene-like layered and wrinkled nanostructure. AFM was utilized to determine the thickness of MoS2 nanosheet. Fig. S2† showed the thickness of MoS2 nanosheet was about 1.14 nm, indicating that the exfoliated MoS2 nanosheet was almost single layer.32,33 From Fig. 2B–D, it can be seen that 20 nm spherical Au nanoparticles, 21 nm spherical Pt nanoparticles and 19 nm core–shell Au–Pt nanoparticles were uniformly supported on the surface of MoS2 nanosheet, respectively. These TEM images suggested that the expected Au–MoS2, Pt–MoS2 and Au@Pt–MoS2 nanocomposites have been successfully synthesized.3.2. Electrochemical characterization of MoS2-based modified electrodes
Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) techniques were used to investigate the electron transfer properties of different MoS2-based modified electrodes. As shown in Fig. 3A, MoS2/GCE (curve b) showed a similar redox peaks with larger currents to the bare GCE (curve a), which ascribed to the large surface area of MoS2 nanosheet. When Au, Pt and Au@Pt nanoparticles decorated on the surface of MoS2 nanosheet, both anodic and cathodic peak currents obviously increased at Au–MoS2/GCE (curve c), Pt–MoS2/GCE (curve d) and Au@Pt–MoS2/GCE (curve e). Moreover, the peak-to-peak potential separation (ΔEp) of Au–MoS2/GCE, Pt–MoS2/GCE and Au@Pt–MoS2/GCE were 93 mV, 96 mV and 101 mV, respectively, which was smaller than that of bare GCE (151 mV). These results indicated that Au–MoS2, Pt–MoS2 and Au@Pt–MoS2 nanocomposites possessed excellent conductivity due to the synergistic effect of MoS2 nanosheet and noble metallic nanoparticles, which greatly enhanced the electron transfer between redox probe and electrode surface. It was noted that Au@Pt–MoS2/GCE had better electrochemical behavior than Au–MoS2/GCE and Pt–MoS2/GCE, ascribing to the dendritic-like structures of Au@Pt and the better electrocatalytic ability of Au@Pt–MoS2 nanocomposites. As we all know, MoS2 has high surface-to-volume ratio and noble metallic nanoparticles has wonderful electrical conductivity and electrocatalytic ability. Therefore, these MoS2-based nanocomposites can efficiently facilitate the electron transfer between the redox probe and the electrode surface. The capacity of electron transfer at different electrodes was also investigated by EIS (Fig. 3B). The electron transfer resistance (Rct) of the MoS2/GCE (curve b) was much larger than that of the bare GCE (curve a). A large number of negative charges on the surface of MoS2, which could hinder the electron transfer between the [Fe(CN)6]3−/4− and the electrode surface due to the electrostatic repulsion. Expectedly, once Au, Pt and Au–Pt nanoparticles decorated on the surface of MoS2 nanosheet, the conductivity of MoS2-based nanohybrids were significantly improved, resulting in the Rct of Au–MoS2/GCE (curve c), Pt–MoS2/GCE (curve d) and Au@Pt–MoS2/GCE (curve e) were obviously smaller than MoS2/GCE. It should be pointed out that the Rct of Au@Pt–MoS2/GCE was smaller than that of Au–MoS2/GCE and Pt–MoS2/GCE, which was consistent with the CV curves. These experimental results suggested MoS2-based nanocomposites possessed excellent conductivity, which could be used as sensing platform in electrochemistry field. To prove the repeatability of these MoS2-based modified electrodes, at least three parallel electrodes were employed. The redox peak currents and Rct of different MoS2-based modified electrodes were listed in Fig. S3,† suggesting the as-prepared electrochemical platforms possessed excellent repeatability.3.3. Electrochemical behavior of CC at MoS2-based modified electrodes
Fig. 4 demonstrated the CVs of the bare GCE, MoS2/GCE, Au–MoS2/GCE, Pt–MoS2/GCE and Au@Pt–MoS2/GCE in 0.1 M PB (pH 7.0) containing 400 μM catechol, respectively. A pair of irreversible redox peaks of CC was obtained at the bare GCE (curve a). The anodic and cathodic peaks of CC were at 334 mV and 24 mV, respectively, leading to the ΔEp was 310 mV. When MoS2 modified on the bare GCE, the anodic and cathodic peak currents were slightly increased and the ΔEp decreased to 191 mV (curve b), suggesting MoS2 nanosheet could improve the electrochemical reversibility of CC. The ΔEp at Au–MoS2/GCE (curve c), Pt–MoS2/GCE (curve d) and Au@Pt–MoS2/GCE (curve e) were 188 mV, 167 mV and 136 mV, respectively, which were smaller than that at MoS2/GCE and bare GCE. Furthermore, the anodic peak currents of Au–MoS2/GCE, Pt–MoS2/GCE and Au@Pt–MoS2/GCE were 1.15, 1.25 and 1.41 folds than that of bare GCE, respectively. These results indicated that such noble metallic nanoparticles-decorated MoS2 nanocomposites possessed strong electrocatalytic activity towards the redox reaction of CC. It also proved that bimetallic nanoparticle-decorated MoS2 nanocomposites possessed better electrochemical performance than single metallic nanoparticles-decorated MoS2 nanocomposites. Similarly, the reproducibility of MoS2-based modified electrodes electrocatalytic ability towards CC was shown in Fig. S4.† To further prove the performance of noble metallic nanoparticles-decorated MoS2 nanocomposites, Au, Pt and Au@Pt nanoparticles were synthesized to determine CC under the same condition. The TEM images of Au, Pt and Au@Pt nanoparticles and the electrochemical performances of Au/GCE, Pt/GCE and Au@Pt/GCE were shown in Fig. S5 and S6,† respectively. The experimental results also confirmed that the MoS2-based nanocomposites had better electrochemical performances due to their synergistic effect. As we know, CC is an isomer of dihydroxybenzenes, which is easily adsorbed and aggregated on the surface of MoS2 nanosheet vis π–π interaction. Therefore, the synergistic effect of the large surface of MoS2 nanosheet and excellent electrocatalytic ability of noble metallic nanoparticles resulted in excellent electrochemical performance. | ||
| Fig. 4 Cyclic voltammograms of (a) bare GCE, (b) MoS2/GCE, (c) Au–MoS2/GCE, (d) Pt–MoS2/GCE and (e) Au@Pt–MoS2/GCE in 0.1 M PB (pH 7.0) solution containing 400 μM CC. Scan rate: 100 mV s−1. | ||
3.4. Effect of scan rate and pH value
The effect of scan rate on the redox peak currents of CC at different modified electrodes were investigated. As shown in Fig. S7,† both anodic and cathodic peak currents increased with the scan rate increasing. Moreover, the anodic peak currents of MoS2/GCE, Au–MoS2/GCE, Pt–MoS2/GCE and Au@Pt–MoS2/GCE were linear with the square root of the scan rate ranging from 2 mV s−1 to 200 mV s−1, suggesting that the reaction of CC at different MoS2-based modified electrodes was a typical diffusion-controlled process (Fig. 5A).34 The influence of pH value on the redox reaction of CC at different modified electrodes were also studied in the range of pH 6.0–8.0. Fig. S8† showed the redox peak potentials of CC at MoS2-based modified electrodes shifted negatively with the pH value increased. The formal potential was linearly proportional to the pH value (Fig. S9†), suggesting two protons coupled with two electrons involved in the reaction process. More importantly, the anodic peak currents of MoS2/GCE, Au–MoS2/GCE, Pt–MoS2/GCE and Au@Pt–MoS2/GCE were largest when pH value was equal to 7.0 (Fig. 5B). Considering the determination sensitivity, pH 7.0 was chosen as the optimal condition in following experiments. | ||
| Fig. 5 Effect of (A) scan rate and (B) pH value on the peak currents of (a) MoS2/GCE, (b) Au–MoS2/GCE, (c) Pt–MoS2/GCE and (d) Au@Pt–MoS2/GCE in the presence of 400 μM CC. | ||
3.5. Electrochemical determination of CC at MoS2-based modified electrodes
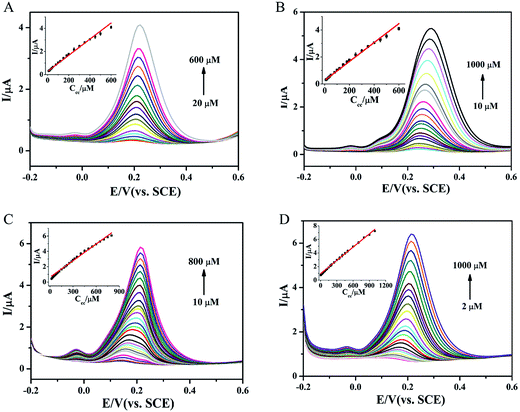
DPV was performed to accurately determine CC at different MoS2-based modified electrodes. As shown in Fig. 6A, the oxidation peak current of CC was increased with the increasing CC concentration at MoS2/GCE. The oxidation peak current of was proportional to the concentration of CC in the range from 20 to 600 μM (inset in Fig. 6A). The regression equation was obtained as Ipc (μA) = 0.0057c (μM) + 0.3767 (R2 = 0.9991) and the detection limit was estimated to be 0.6 μM (S/N = 3). Similarly, the oxidation peak currents of Au–MoS2/GCE, Pt–MoS2/GCE and Au@Pt–MoS2/GCE were linear with the CC concentration ranging from 10 μM to 1000 μM, 10 μM to 800 μM and 2 μM to 1000 μM with estimated detection limits of 0.57 μM, 0.47 μM and 0.44 μM, respectively (Fig. 6B–D). These linear ranges and detection limits of different MoS2-based electrodes were comparable to or better than some nanomaterials-based electrodes, which is shown in Table S1.† From the results, it can be found that noble metallic nanoparticles-decorated MoS2 nanocomposites exhibited better electrochemical performance than MoS2 nanosheet, including linear range and detection limit. It was also noted that the bimetallic nanoparticles-decorated MoS2 nanocomposite (Au@Pt–MoS2) showed better performance (wider linear range) than single nanoparticles-decorated MoS2 nanocomposites (Au–MoS2 and Pt–MoS2), which was consistent with the result in Fig. 3.For further application, the stability of these MoS2-based electrochemical sensors should be investigated. As shown in Fig. S10 and S11,† the cyclic stability and long-term stability of these MoS2-based sensors were studied. The peak currents of MoS2-based sensors could retain over 70% after 100 successively scanning and 20 days storage, suggesting that such MoS2-based sensors had excellent stability.
3.6. Analytical applications
To explore the feasibility of MoS2-based sensing platforms to detect CC in practice, CC was added into river water and local tap water to simulate actual samples. The founded amount and recovery of CC detection were summarized in Table 1. The recovery were varied from 96.6% to 105.4% and relative standard deviation (RSD) was less 5% for MoS2-based electrodes, suggesting these MoS2-based electrochemical sensing platforms could be used to determine CC in real samples.| Materials | CC added (μM) | River water | Local tap water | ||||
|---|---|---|---|---|---|---|---|
| CC founded (μM) | Recovery (%) | RSD (%) | CC founded (μM) | Recovery (%) | RSD (%) | ||
| MoS2 | 1 | 1.03 | 103.2 | 2.12 | 1.04 | 104.1 | 3.24 |
| 2 | 1.99 | 99.4 | 2.51 | 1.95 | 97.7 | 1.93 | |
| 5 | 5.01 | 100.2 | 1.31 | 4.85 | 96.9 | 2.23 | |
| Au–MoS2 | 1 | 1.05 | 105.4 | 3.01 | 1.03 | 103.4 | 1.39 |
| 2 | 1.98 | 98.9 | 1.73 | 1.04 | 103.6 | 2.74 | |
| 5 | 4.83 | 96.6 | 2.22 | 4.85 | 96.9 | 0.98 | |
| Pt–MoS2 | 1 | 1.03 | 103.7 | 2.63 | 0.99 | 99.1 | 3.42 |
| 2 | 2.03 | 102.1 | 4.78 | 2.02 | 101.3 | 2.94 | |
| 5 | 5.17 | 103.4 | 3.84 | 5.06 | 101.1 | 1.49 | |
| Au@Pt–MoS2 | 1 | 1.02 | 102.4 | 1.94 | 1.03 | 102.9 | 3.97 |
| 2 | 2.06 | 103.1 | 2.91 | 2.03 | 101.6 | 1.89 | |
| 5 | 4.83 | 96.6 | 0.96 | 4.92 | 98.5 | 2.06 | |
4. Conclusion
In this work, CC was chosen as analyte to investigate the electrochemical performances of MoS2 nanosheet and three MoS2-based nanocomposites. If combining the advantageous characteristics of MoS2 and noble metallic nanoparticles, the nanohybrids exhibited better electrochemical performance than pure MoS2 nanosheet and noble metallic nanoparticles. For example, Au–MoS2, Pt–MoS2 and Au@Pt–MoS2 modified electrodes exhibited a wider detection range and a lower detection limit than MoS2/GCE under the same experimental condition. Moreover, these MoS2-based electrochemical sensors could be employed to determine CC in water samples with satisfactory results. All the results suggested that properties of MoS2-based nanocomposites can be tuned according to the natural properties of MoS2 nanosheet and other components. A MoS2-based nanocomposite may be a promising candidate for the future study of electrochemical sensors for chemical and biological molecules detection.Acknowledgements
This work was financially supported by the National Basic Research Program of China (2012CB933301), the National Natural Science Foundation of China (21305070, 21475064), the Natural Science Foundation of Jiangsu Province (BK20130861), the Sci-tech Support Plan of Jiangsu Province (BE2014719), the Program for Changjiang Scholars and Innovative Research Team in University (IRT_15R37), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, YX03001).Notes and references
- M. Chhowalla, H. S. Shin, G. Eda, L. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed
.
- X. Huang, C. Tan, Z. Yin and H. Zhang, Adv. Mater., 2014, 26, 2185–2204 CrossRef CAS PubMed
.
- H. Li, G. Lu, Z. Yin, Q. He, H. Li, Q. Zhang and H. Zhang, Small, 2012, 8, 682–686 CrossRef CAS PubMed
.
- O. Lopez-Sanchez, D. Lembke, M. Kayci, A. Radenovic and A. Kis, Nat. Nanotechnol., 2013, 8, 497–501 CrossRef CAS PubMed
.
- C. Tan and H. Zhang, Chem. Soc. Rev., 2015, 44, 2713–2731 RSC
.
- G. Eda, H. Yamaguchi, D. Voiry, T. Fujita, M. Chen and M. Chhowalla, Nano Lett., 2011, 11, 5111–5116 CrossRef CAS PubMed
.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147–150 CrossRef CAS PubMed
.
- A. Splendiani, L. Sun, Y. Zhang, T. Li, J. Kim, C. Y. Chim, G. Galli and F. Wang, Nano Lett., 2010, 10, 1271–1275 CrossRef CAS PubMed
.
- H. Wang, L. Yu, Y. H. Lee, Y. Shi, A. Hsu, M. L. Chin, L. J. Li, M. Dubey, J. Kong and T. Palacios, Nano Lett., 2012, 12, 4674–4680 CrossRef CAS PubMed
.
- T. F. Jaramillo, K. P. Jergensen, J. Bonde, J. H. Nielsen, S. Horch and I. Chorkendorff, Science, 2016, 317, 100–102 CrossRef PubMed
.
- J. Chao, X. Han, H. Sun, S. Su, L. Weng and L. Wang, Sci. China: Chem., 2016, 59, 332–337 CrossRef CAS
.
- S. Su, H. Sun, F. Xu, L. Yuwen, C. Fan and L. Wang, Microchim. Acta, 2014, 181, 1497–1503 CrossRef CAS
.
- S. Wu, Z. Zeng, Q. He, Z. Wang, S. J. Wang, Y. Du, Z. Yin, X. Sun, W. Chen and H. Zhang, Small, 2012, 8, 2264–2270 CrossRef CAS PubMed
.
- C. Zhu, Z. Zeng, H. Li, F. Li, C. Fan and H. Zhang, J. Am. Chem. Soc., 2013, 135, 5998–6001 CrossRef CAS PubMed
.
- J. Xiao, D. Choi, L. Cosimbescu, P. Koech, J. Liu and J. P. Lemmon, Chem. Mater., 2010, 22, 4522–4524 CrossRef CAS
.
- T. Wang, R. Zhu, J. Zhuo, Z. Zhu, Y. Shao and M. Li, Anal. Chem., 2014, 86, 12064–12069 CrossRef CAS PubMed
.
- K. Chang and W. Chen, ACS Nano, 2011, 5, 4720–4728 CrossRef CAS PubMed
.
- S. Bertolazzi, D. Krasnozhon and A. Kis, ACS Nano, 2013, 7, 3246–3252 CrossRef CAS PubMed
.
- Q. Xiang, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2012, 134, 6575–6578 CrossRef CAS PubMed
.
- X. Huang, Z. Zeng, S. Bao, M. Wang, X. Qi, Z. Fan and H. Zhang, Nat. Commun., 2013, 4, 1444 CrossRef PubMed
.
- L. Yuwen, F. Xu, B. Xue, Z. Luo, Q. Zhang, B. Bao, S. Su, L. Weng, W. Huang and L. Wang, Nanoscale, 2014, 6, 5762–5769 RSC
.
- S. Su, C. Zhang, L. Yuwen, X. Liu, L. Wang, C. Fan and L. Wang, Nanoscale, 2015, 8, 602–608 RSC
.
- C. Kuru, C. Choi, A. Kargar, D. Choi, Y. J. Kim, C. H. Liu, S. Yavuz and S. Jin, Adv. Sci., 2015, 2, 1500004 CrossRef
.
- J. Lu, J. H. Lu, H. Liu, B. Liu, L. Gong, E. S. Tok, K. P. Loh and C. H. Sow, Small, 2015, 11, 1792–1800 CrossRef CAS PubMed
.
- S. Su, C. Zhang, L. Yuwen, J. Chao, X. Zuo, X. Liu, C. Song, C. Fan and L. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 18735–18741 CAS
.
- J. Chao, M. Zou, C. Zhang, H. Sun, D. Pan, H. Pei, S. Su, L. Yuwen, C. Fan and L. Wang, Nanotechnology, 2015, 26, 274005 CrossRef PubMed
.
- S. Su, H. Sun, F. Xu, L. Yuwen and L. Wang, Electroanalysis, 2013, 25, 2523–2529 CrossRef CAS
.
- S. Su, M. Zou, H. Zhao, C. Yuan, Y. Xu, C. Zhang, L. Wang, C. Fan and L. Wang, Nanoscale, 2015, 7, 19129–19135 RSC
.
- H. Sun, J. Chao, X. Zuo, S. Su, X. Liu, L. Yuwen, C. Fan and L. Wang, RSC Adv., 2014, 4, 27625 RSC
.
- W. Liu, L. Wu, X. Zhang and J. Chen, Anal. Methods, 2014, 6, 718–724 RSC
.
- M. Nazari, S. Kashanian and R. Rafipour, Spectrochim. Acta, Part A, 2015, 145, 130–138 CrossRef CAS PubMed
.
- C. Lee, H. Yan, L. E. Brus, T. F. Heinz, J. Hone and S. Ryu, ACS Nano, 2010, 4, 2695–2700 CrossRef CAS PubMed
.
- R. S. Sundaram, M. Engel, A. Lombardo, R. Krupke, A. C. Ferrari, P. Avouris and M. Steiner, Nano Lett., 2013, 13, 1416–1421 CrossRef CAS PubMed
.
- S. M. Ghoreishi, M. Behpour and M. H. M. Fard, J. Solid State Electrochem., 2011, 16, 179–189 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12401a |
| This journal is © The Royal Society of Chemistry 2016 |