Zwitterionic polymer brush coatings with excellent anti-fog and anti-frost properties†
Abstract
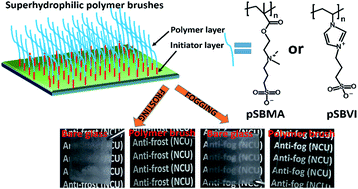
The formation of fog and frost on transparent surfaces can lead to many problems in our daily life. To address these problems, polymer brushes based on two zwitterionic analogues, poly(sulfobetaine methacrylate) (pSBMA) and poly(sulfobetaine vinylimidazole) (pSBVI), have been prepared by surface-initiated atom transfer radical polymerization (SI-ATRP). Hydrophilic and superhydrophilic pSBMA and pSBVI polymer brushes were prepared by controlling the thickness of the coatings to study the effect of wettability on the anti-fog and anti-frost properties. X-ray photoelectron spectroscopy (XPS), ellipsometry and atomic force microscopy (AFM) were respectively used to determine the interfacial elemental composition, the thickness and the morphology of the brushes. The wettability of the polymer brushes was measured using a water contact angle goniometer. Their anti-fog and anti-frost capabilities were determined visually and tested quantitatively by UV-vis spectroscopy. The results indicated that the optical transmittance of substrates modified with superhydrophilic polymer coatings under both hot and cold fogging conditions was very high. Additionally, no visible frost was formed on the superhydrophilic substrates after storage in a freezer at −20 °C for the duration of the experiment. These results convincingly demonstrated that the resistance of the modified substrates to fogging and frosting is strongly correlated to the surface wettability. Moreover, there is no considerable difference in the performance of pSBMA and pSBVI polymer brushes, but the former is preferred because SBMA monomer is commercially available and requires short polymerization time to obtain superhydrophilicity.

 Please wait while we load your content...
Please wait while we load your content...