The role of a xylose isomerase pathway in the conversion of xylose to lipid in Mucor circinelloides
Abstract
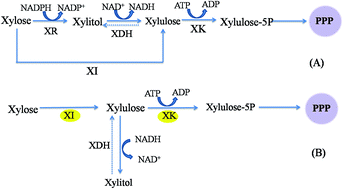
The xylose isomerase (XI) pathway, which converts xylose in lignocellulosic materials into intermediate metabolites, does not commonly exist in filamentous fungi. The genomic sequence of Mucor circinelloides shows that it has the XI pathway but it has never been characterized. In this study, the genes coding for XI and xylulokinase (XK) were overexpressed in Mucor circinelloides to examine their effects on xylose utilization and the xylose metabolism pathway. The results showed that overexpressing XI or XK increased the total consumption rate of xylose and the lipid yield. In the lipid accumulation phase, the mRNA levels of XI and XK, and activities of XI, XK, glucose 6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGDH) in recombinant strains were higher than the control. Our study suggested that the XI pathway plays an important role in xylose utilization and lipid accumulation in the filamentous fungus Mucor circinelloides.

 Please wait while we load your content...
Please wait while we load your content...