Flexible and durable cellulose aerogels for highly effective oil/water separation
Abstract
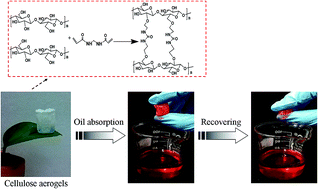
In this paper, a facile and green method was presented to prepare flexible, ultralight, and hydrophobic cellulose aerogels (CA) on the chemical cross-linking of cellulose solution, lyophilization and subsequent hydrophobic modification with methyltrichlorosilane (MTCS) by a thermal chemical vapor deposition (CVD) process. The resultant cellulose aerogels had an interconnected and highly porous structure with a varied pore size owing to different starting solution concentrations. And also the cellulose aerogels were ultralight (as low as 0.027 g cm−3), but they exhibited improved mechanical properties with compress stress of 1.10–3.85 MPa. After silanization, the MTCS-modified cellulose aerogels showed durable hydrophobicity with an average water contact angle of 141°, even maintaining 131° after 5 days. Furthermore, the flexible and hydrophobic cellulose aerogels could rapidly collect oils and organic solvents both on the surface and bottom from water, and exhibited excellent adsorption performances (e.g. 59.32 g g−1 of pump oil) as well as good recyclability. Thus, such a flexible and hydrophobic cellulose aerogel is a very promising material for oil spill cleanup and industrial oily wastewater purification.

 Please wait while we load your content...
Please wait while we load your content...