Efficient and stable polymer solar cells with electrochemical deposition of CuSCN as an anode interlayer†
Abstract
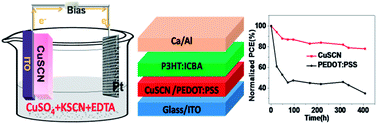
Electrochemical deposition (ECD) of a copper thiocyanate (CuSCN) film as an efficient anode interlayer (AIL) in polymer solar cells (PSCs) is reported. By elaborately optimizing the ECD conditions for the preparation of CuSCN films, a power conversion efficiency (PCE) of 6.16% has been achieved through employing P3HT : IC60BA as the active layer. Concerning the long-term stability, the PSCs with CuSCN as the AIL maintain nearly 84% of their initial PCEs after storing in ambient conditions for 400 hours without any encapsulation, while the PCEs of the reference PSCs with PEDOT:PSS as the AIL largely decrease to 35% of the initial values. Due to the advantages of the high hole mobility and superior chemical stability, ECD-prepared CuSCN is a promising alternative to PEDOT:PSS as the AIL for PSCs.


 Please wait while we load your content...
Please wait while we load your content...