Ultrathin polymer gel-infiltrated monolayer colloidal crystal films for rapid colorimetric chemical sensing
Abstract
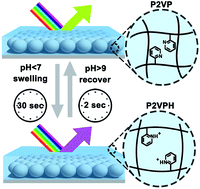
A new optical motif compatible with an ultrathin polymer gel film for chemical sensing was fabricated by infiltrating polymer gel into an inactive scaffold of monolayer colloidal crystals. The composite film, denoted as PG-MCC, features submicron thickness and presents thin film interference with only one reflectance peak in the visible region because of appropriate optical thickness. Consequently, the film exhibits distinct reflective color, which can be easily tuned by adjusting the fabrication parameters. In this study, PG-MCC was demonstrated as a pH sensor by using a weak polyelectrolyte poly-(2-vinyl pyridine) (P2VP). The pH-induced swelling and deswelling of P2VP gel led to a substantial thickness change and hence a reflectance peak shift of PG-MCC. Highly reversible, linear, reliable and very fast responses to pH variations were exhibited. Moreover, a colorimetric readout was readily achieved, making it promising for continuous monitoring of environmental analytes by the naked eye.

 Please wait while we load your content...
Please wait while we load your content...