Physicochemical properties of functionalized 1,3-dialkylimidazolium ionic liquids based on the bis(fluorosulfonyl)imide anion†
Guojun Wanga,
Shaohua Fang*ac,
Yi Liua,
Dong Luoa,
Li Yang*abc and
Shin-ichi Hiranob
aSchool of Chemistry and Chemical Engineering, Shanghai Jiaotong University, Shanghai 200240, China. E-mail: housefang@sjtu.edu.cn; liyangce@sjtu.edu.cn; Fax: +86 21 54741297; Tel: +86 21 54748917
bHirano Institute for Materials Innovation, Shanghai Jiaotong University, Shanghai 200240, China
cShanghai Electrochemical Energy Devices Research Center, Shanghai 200240, China
First published on 4th July 2016
Abstract
A new series of ether- or alkenyl-functionalized 1,3-dialkylimdazolium ILs based on the FSI anion were prepared and their physicochemical properties (melting point, thermal stability, viscosity, conductivity and electrochemical stability) were studied in detail and compared with the corresponding TFSI-based ILs. It was confirmed that introduction of ether or alkenyl groups and FSI anions jointly could reduce viscosity and enhance conductivity. These FSI-based ILs owned viscosities lower than 30 mPa s and conductivities higher than 7 mS cm−1. AEI-FSI had the lowest viscosity (17.4 mPa s) among all the reported FSI-based ILs and it had relatively higher conductivity (12.8 mS cm−1) as well. The electrochemical windows of most ILs were wider than 3.7 V, indicating their promising application for electrochemical devices.
1. Introduction
Ionic liquids (ILs) are molten salts composed of organic cations and different anions entirely. Attributed by a suite of unique properties such as non-flammability, negligible volatility and high thermal stability,1,2 ILs have been used as important industrial solvents for separation processes,3,4 catalysis5,6 and potential electrolytes for various electrochemical devices.7–10 Particularly, a large number of investigations have focused on ILs as novel safe electrolytes for rechargeable lithium-ion batteries because they can reduce the safety risk caused by conventional organic electrolytes.11–14The representative cations utilized in ionic liquids are quaternary ammonium species such as tetraalkylammonium, pyrrolidinium, piperidinium, imidazolium and pyrazolium families. Thereinto, 1,3-dialkylimidazolium ILs have been researched more widely since their relative low viscosity, high conductivity and toilless synthesis.15–18 Among this family, 1-ethyl-3-methylimidazolium (EMI) is regarded as the typical cation. Integrating low molecular weight and sufficient side chain mobility, EMI cation can endow ILs with lower viscosity compared to other 1,3-dialkylimidazolium ILs with longer alkyl side chains.19 A series of lower viscous ILs comprised of EMI cation and different anions have been synthesized, such as EMI dicyanamide (EMI-DCA) and EMI 2,2,2-trifluoro-N-(trifluoromethylsulfonyl)acetamide (EMI-TSAC). And the viscosities of these ILs are all less than 25 mPa s at ambient temperature.20–22
Up to now, the high viscosity of ILs still restricts their applications as contrasted with that of organic solvents. Fortunately another remarkable advantage of ILs is that their physical and electrochemical properties can be changed easily by diverse structural variation due to the designability of cations and anions. Introducing a functional group into cation is a commonly used method to obtain new ILs.23 However, it has been verified that electron-withdrawing groups (i.e. ester group, phenyl group, cyano group) can result in increment of viscosity.24–28 In contrast, ether group owning electron-donating effect can decrease viscosity and melting point but not induce to the obvious deterioration of thermal and electrochemical stability meanwhile.29–33 So far, many different ether-functionalized cations, such as pyrrolidinium,29 piperidinium,29,34 imidazolium30,35,36 and tetraalkylammonium,31,37 have been reported and show superior characteristics. Recently, alkenyl functionalization has also attracted much interest.38–41 Mizumo et al. have synthesized a serious of allylimidazolium halides with lower melting point.39 Young et al. have reported that allyl-substituted ILs demonstrated higher conductivity and lower viscosity compared with fully saturated analogues.41,42 Even though the mechanism about how the carbon–carbon double bond of alkenyl group influences physicochemical properties is not so clear now, many researchers have proposed that the increased π–π interactions can lead to better properties.40,42–44
During the last decade, the most popular anion used in ILs is bis(trifluoromethanesulfonyl)imide (TFSI) anion, because of its highly delocalized charge distribution, high flexibility and good thermal and electrochemical stability.45 Besides TFSI anion, another fluorinated sulfonyl type anions, bis(fluorosulfonyl)imide (FSI) anion has also gained significant attention. Compared with the respective TFSI counterparts, many oniums (i.e. imidazolium,21,46 pyrrolidinium,21,47 phosphonium48,49) with FSI anion can exhibit lower viscosity and higher conductivity. Moreover, it has been accepted that the FSI-based ILs as electrolytes of lithium-ion batteries can show better compatibility with graphite negative electrode.50–52 In order to understand the superiority of FSI-based ILs, numerous comparative investigations have been conducted to clarify the effect of FSI and TFSI anions on the physicochemical and electrochemical properties of ILs.46–49,53,54 Tsuzuki et al. have explained that weaker interactions between cations and FSI anions can result in lower viscosity.53
Thus far, some ILs based on functionalized cations and FSI anion have been reported detailedly, including ether-functionalized tetraalkylammonium,37 ether- or alkenyl-functionalized phosphonium,48,49 and ether-, ester-, or cyano-functionalized sulfonium ILs,25 but the representative 1,3-dialkylimidazoulium ILs are just proposed in a patent.55 In this work, we synthesized a series of ether- or alkenyl-functionalized 1,3-dialkylimidazolium ILs based on FSI anion. And the structures of these FSI-based ILs were shown in Fig. 1. The thermal properties, viscosity, conductivity and electrochemical stability of these FSI-based ILs were investigated systematically and compared with their TFSI-based counterparts. The viscosities of these FSI-based ILs were all lower than 30 mPa s at room temperature. To our knowledge, AEI-FSI had the lowest viscosity among the reported FSI-based ILs.
2. Experimental
2.1 Synthesis of functionalized 1,3-dialkylimidazolium ILs
1-Vinyl-3-ethylimidazolium bis(fluorosulfonyl)imide (VEI-FSI) was chosen as an example to illustrate the process of synthesis. 1-Vinylimidazole (5 g, 0.053 mol) and bromoethane (6.95 g, 0.064 mol) were reacted in a 250 mL flask at 30 °C for 24 h with acetonitrile (10 mL) as the solvent. The product was washed with diethyl ether (100 mL) three times. Then the crude bromide was purified by active carbon with ethanol (100 mL) as the solvent for 24 h. After removing active carbon and ethanol by filtration and rotary evaporation respectively, the bromide was dissolved in deionized water with same molar amount lithium bis(fluorosulfonyl)imide (LiFSI) and stirred for 4 h at ambient temperature. Dichloromethane was used to extract the IL from the mixture and then the solvent was washed with deionized water for 3 times until no halide residual could be detected by AgNO3 solution. The dichloromethane was removed by rotary evaporation. The product was dried under high vacuum for more than 10 h at 100 °C.Detailed procedures and NMR data of 1H and 13C were described in ESI.†
2.2 Measurement
1H NMR and 13C NMR spectra (Bruker, Advance III HD 400) were utilized to verify the structure of synthesized ILs. A moisture titrator (Metrohm 73KF Karl Fischer coulometer) was used to detect the water contents of ILs, which were all below 50 ppm.The phase transition behavior of ILs was analyzed by using a differential scanning calorimeter (DSC, Perkin Elmer 8000). Each IL (5 mg approximately) sealed well by a small aluminum crucible under dry atmosphere. Firstly, the sample was cooled to −60 °C from ambient temperature and held for 10 min to guarantee its absolute crystallization (if possible). Then the sample was heated and cooled at a scan rate of 10 °C min−1 from −60 °C to 40 °C. The above procedures were repeated twice and then the thermal data of the second heating-cooling scan were collected. Thermal stability was tested by thermal gravimetric analysis (TGA, TA Instrument Q5000). The sample was dropped into a platinum pan and then heated to 600 °C at the rate of 10 °C min−1 under nitrogen atmosphere.
The density was measured by weighing each IL (1.00 mL) in an argon-filled glove box at 25 °C. The viscosity was tested by a Brookfield viscometer (DV-III) and the conductivity was determined by a conductivity meter (DDS-11A). The values of viscosity and conductivity were recorded every 5 °C in the temperature range from 25 °C to 80 °C (Brookfield temperature controller, TC-502). The electrochemical window was analyzed by means of linear sweep voltammetry (LSV) through an electrochemical workstation (CHI-600D) in the glove box. The working electrode, reference electrode and counter electrode were glassy carbon electrode (3 mm diameter), sliver electrode and platinum electrode respectively. The positive and negative scans were carried out separately using neat IL. After each scan, the glassy carbon electrode was polished by nano-alumina powder and washed by deionized water.
3. Results and discussion
3.1 Thermal properties
The physicochemical properties of 8 FSI-based 1,3-dialklimidazolium and the corresponding TFSI-based ILs were summarized in Table 1, including melting point, density, thermal decomposition temperature, viscosity and conductivity.| Ionic liquids | Mwa/g mol−1 | Tmb/°C | dc/g cm−3 ±5% | ηd/mPa s ±5% | σe/mS cm−1 ±5% | Tdf/°C |
|---|---|---|---|---|---|---|
| a Molecular weight.b Melting point noted from the onset.c Density at 25 °C.d Viscosity at 25 °C.e Conductivity at 25 °C.f Decomposition temperature of 10% weight loss.g Ref. 36. | ||||||
| VEI-FSI | 303.4 | −23 | 1.31 | 22.9 | 11.1 | 249.4 |
| AMI-FSI | 303.4 | −3 | 1.31 | 19.3 | 11.2 | 248.2 |
| AEI-FSI | 317.4 | <−60 | 1.29 | 17.4 | 12.8 | 257.4 |
| Im2o1-1-FSI | 321.4 | <−60 | 1.40 | 28.6 | 8.12 | 233.4 |
| Im2o1-2-FSI | 335.4 | <−60 | 1.37 | 24.0 | 8.64 | 239.9 |
| Im2o2-1-FSI | 335.4 | <−60 | 1.35 | 28.3 | 7.13 | 228.7 |
| Im2o2-2-FSI | 349.4 | <−60 | 1.31 | 24.4 | 7.76 | 231.8 |
| EMI-FSI | 291.4 | −20 | 1.43 | 18.3 | 14.3 | 258.6 |
| VEI-TFSI | 403.4 | 4 | 1.46 | 46.5 | 6.16 | 376.8 |
| AMI-TFSI | 403.4 | <−60 | 1.48 | 31.0 | 7.35 | 383.8 |
| AEI-TFSI | 417.4 | <−60 | 1.44 | 28.2 | 7.65 | 357.3 |
| Im2o1-1-TFSIg | 421.4 | <−60 | 1.45 | 41.8 | 4.62 | 389.8 |
| Im2o1-2-TFSIg | 435.4 | <−60 | 1.39 | 35.9 | 5.12 | 381.5 |
| Im2o2-1-TFSIg | 435.4 | <−60 | 1.39 | 34.8 | 4.37 | 394.1 |
| Im2o2-2-TFSIg | 449.4 | <−60 | 1.36 | 31.3 | 4.68 | 374.9 |
| EMI-TFSIg | 391.4 | −17 | 1.51 | 34.1 | 8.58 | 407.7 |
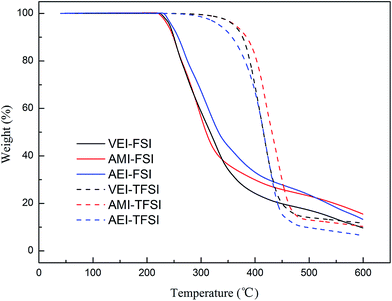
The DSC curves of 3 FSI-based alkenyl-functionalized ILs were illustrated in Fig. 2 as examples. AMI-FSI and VEI-FSI showed one melting transition (Tm). AEI-FSI didn't display any phase transition behavior in the range of −60 °C to 40 °C and thus its melting point was defined as “<−60 °C” according to the previous papers.32,56,57 And four FSI-based ether-functionalized ILs had the same result of DSC test as AEI-FSI.
In general, the melting point of ILs was mainly determined by three factors: ion asymmetry, interactions between cation and anion, and conformational freedom of ion. Functionalization of cation would drastically influence the melting point. As well known, ether-functionalization of cation could be helpful to decrease the melting point, due to high flexibility of ether group, asymmetry of cation, and reduced electrostatic forces between cation and anion as the result of electron-donating effect of ether group.32,33,37,58 According to Table 1, all the ether-functionalized 1,3-dialklimidazolium ILs possessed the melting point lower than −60 °C, no matter whether the anion was TFSI or FSI. By contrast, the effect of alkenyl-functionalization seemed more complicated. For TFSI-based ILs, high asymmetry of cation was favorable to lower melting point. So the melting point of EMI-TFSI (−17 °C) were higher than those of AMI-TFSI and AEI-TFSI (<−60 °C) but lower than that of VEI-TFSI (4 °C). For FSI-based ILs, the changing rule of melting point was not consistent with the asymmetry of cation. The melting point of AMI-FSI (−3 °C) was even higher than that of EMI-FSI (−20 °C). It was inferred that the more complex ionic association of AMI-FSI might result in a relatively lower conformational freedom and cause the higher melting point.
The TGA traces of 3 FSI-based alkenyl-functionalized ILs and their corresponding TFSI ILs were shown in Fig. 3 as examples. As summarized in Table 1, the decomposition temperatures of the FSI-based ILs were in the range of 220–250 °C while those of the TFSI-based ILs were above 350 °C. So these FSI-based ILs were not as thermally stable as their TFSI analogues, which was consistent with other kinds of cation including phosphonium,49 tetraalkylammonium37 and pyrrolidinium.59 This was presumably resulted from that the FSO2-group of FSI anion was more inclined to pyrolysis.60 Moreover, different kinds of cations would impact the thermal stability of the FSI-based ILs. The decomposition temperatures of these 1,3-dialkylimdazolium ILs were closed to those of sulfonium (200–270 °C) ILs but lower than those of phosphonium (280–310 °C) and tetraalkylammonium (280–310 °C) ILs.25,37,49 It was also found that the introduction of alkenyl or ether group into 1,3-dialkylimidazolium cations would slightly weaken the thermal stability in different extent, which had been similarly seen in sulfonium and tetraalkylammonium ILs.25,29 Nevertheless, the thermal stability of these functionalized 1,3-dialklimidazolium ILs was still remarkably higher than that of conventional organic electrolytes containing high flammable carbonates.61
3.2 Density
For both TFSI-based and FSI-based 1,3-dialklimidazolium ILs, introduction of ether or alkenyl group could decrease the density because the longer side chain of cation was detrimental to dense packing. The density difference among ether-functionalized 1,3-dialklimidazolium ILs could also be ascribed to the alteration of side chain length of cation. For example, the density of these four FSI-based ether-functionalized ILs varied in the order as follows: Im2o2-2-FSI < Im2o2-1-FSI ≈ Im2o1-2-FSI < Im2o1-1-FSI. In accord with tetraalkylammonium,37 phosphonium49 and sulfonium ILs,25,34 the densities of FSI-based 1,3-dialklimidazolium ILs were smaller than those of their TFSI counterparts, for the weaker interactions between cations and FSI anion could conduce to loose packing.3.3 Viscosity
Exploration of low-viscous ILs was one of the crucial issues for electrochemical applications since the viscosity could influence mass transport property significantly.32 In general, the viscosity of IL was affected by ion size, ion complexes and ion interactions (such as van der Waal force, electrostatic as well as H-bonding interactions). It had been proved that the lower viscosity of FSI-based ILs compared with the TFSI analogues was attributed to not only the smaller size of FSI anion but also the weaker interactions of FSI anion with cation.53 In terms of Table 1, the viscosities of 7 new FSI-based ILs were all lower than 30 mPa s at ambient temperature, and the viscosity of AEI-FSI (17.4 mPa s) was even lower than that of EMI-FSI (18.3 mPa s).For TFSI-based ILs, the introduction of allyl group into pyrrolidinium,42 piperidinium,42 and phosphonium43,48 cations would reduce the viscosity. Here, AEI-TFSI and AMI-TFSI had lower viscosities than EMI-TFSI. Fei et al. had indicated that there are intermolecular π–π stacking interactions between the imidazolium rings and allyl groups in AEI and AMI cations, and their low viscosities could be ascribed to the increased π–π interactions at the expense of H-bonding interactions.40 However, VEI-TFSI, which had the smaller size of cation and the conjugation effect between imidazolium ring and vinyl group, owned higher viscosity among them. It could be inferred that strong π–π stacking interactions between the imidazolium rings in VEI cation might influence the fluidity negatively. When TFSI anion was substituted by FSI anion, the advantage of allyl group in viscosity seemed indistinctive and the viscosity of AMI-FSI (19.3 mPa s) was even higher than that of EMI-FSI (18.3 mPa s) at room temperature. This suggested that anion structure would have effect on π–π stacking interactions in cations.
Ether-functionalization of cation was an effective way to reduce the viscosity since the electrostatic interaction between cation and anion could be weakened by the electron-donating effect of ether group.36,48,62 And this rule had been universally confirmed in TFSI-based imidazolium, tetraalkylammonium, phosphonium, morpholinium and guanidinium ILs.32,33,62 Like ether-functionalized tetraalkylammonium and phosphonium ILs, the viscosities of these ether-functionalized 1,3-dialklimidazolium ILs decreased when TFSI anion was replaced by FSI anion. However, compared to EMI cation, these ether-functionalized cations with larger size did not lead to the obvious decline of viscosity. This could be attributed to stronger van der Waal force between ether-functionalized cations and FSI anion.
Fig. 4 illustrated the temperature dependence of viscosity for all the ILs over the range from 25 to 80 °C. The relationship of viscosity values and temperature could be fitted by a Vogel–Tammann–Fulcher (VTF) model, as shown in eqn (1).
 | (1) |
 | ||
| Fig. 4 VTF plots of viscosity: (a) FSI-based ether-functionalized ILs, (b) FSI-based alkenyl-functionalized ILs and EMI-FSI, and (c) TFSI-based alkenyl-functionalized ILs. | ||
3.4 Conductivity
The conductivity of ILs is a vital property for its electrochemical applications. The phenomenon that FSI anion was more beneficial to improve conductivity than TFSI anion, had been widely observed in different kinds of ILs such as pyrrolidinium,21 piperidinium,21 sulfonium25,34 and phosphonium.48 The FSI-based ILs possessed larger self-diffusion coefficients of ions and higher conductivity attributed to smaller size of FSI anion and weaker interactions between cation and FSI anion.53 Besides, by the molecular dynamics simulations of ILs, Borodin et al. proposed that the lower torsional barrier of FSI-based ILs might be another reason of higher conductivity.64 In this work, these FSI-based functionalized 1,3-dialklimidazolium ILs also exhibited high-conductivity characteristic. As listed in Table 1, the conductivities of FSI-based alkenyl-functionalized ILs were all higher than 11 mS cm−1, and the FSI-based ether-functionalized ILs had the conductivities above 7 mS cm−1.Moreover, functionalization of cation was influential to conductivity as well. Since the increment of cation size, these FSI-based functionalized ILs were not as high-conductive as EMI-FSI. For example, AEI-FSI possessed lower viscosity than EMI-FSI, whereas the conductivity of AEI-FSI (12.8 mS cm−1) was still lower than that of EMI-FSI (14.3 mS cm−1). However, compared to AEI-FSI, VEI-FSI owing smaller cation size had lower conductivity. It suggested that the strong π–π stacking interactions between the imidazolium rings in VEI cation hindered the ion mobility and thus caused an adverse effect on conductivity. Among these FSI-based ether-functionalized 1,3-dialklimidazolium ILs, Im2o1-2-FSI had the highest conductivity even though it did not have the smallest cation size. This consequence might be ascribable to the formation of ion complexes or ion clusters, which was another factor in conductivity.65
Variation of conductivity with temperature from 25 to 80 °C was shown in Fig. 5. Likewise, a VTF model (eqn (2)) could be used to describe the relationship of conductivity and temperature, where σ0 (mS cm−1), B (K) and T0 (K) were three adjustable parameters which had similar physical meanings to those in eqn (1).
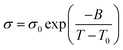 | (2) |
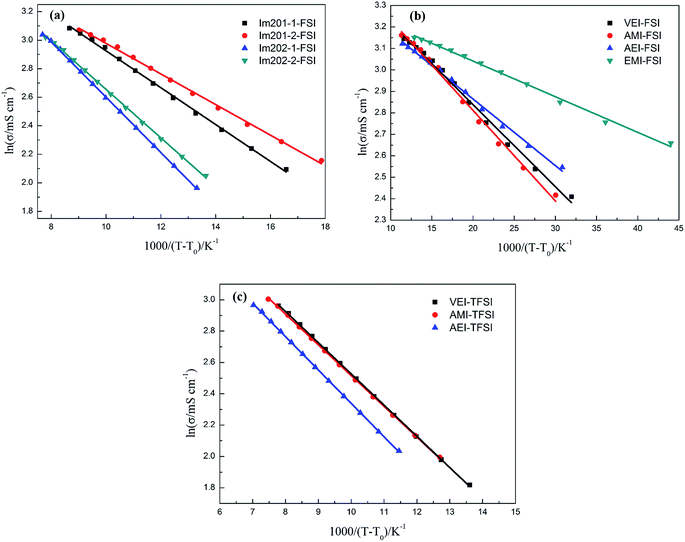 | ||
| Fig. 5 VTF plots of conductivity: (a) FSI-based ether-functionalized ILs, (b) FSI-based alkenyl-functionalized ILs and EMI-FSI, and (c) TFSI-based alkenyl-functionalized ILs. | ||
The values of these parameters and the corresponding fitting coefficient R2 were listed in Table S2† in ESI. ILs with smaller T0 value usually had larger B value. Among these ILs, Im2o2-1-FSI had the smallest T0 value and largest B value whereas EMI-FSI had the largest T0 value and the smallest B value. Additionally, the T0 and B values of viscosity seem to be in inverse proportion to those of conductivity for a certain IL.
3.5 Electrochemical stability
The electrochemical stability was tested via liner sweep voltammetry (LSV). All the LSV curves were illustrated in Fig. 6, and the detailed values of cathodic limiting potential, anodic limiting potential and electrochemical window of each IL were summarized in Table 2. Herein, the electrochemical window of a specific IL was determined by its difference between anodic limiting potential and cathodic limiting potential. For example, the anodic limiting potential of AMI-FSI was around +2.0 V (versus Ag/Ag+) while its cathodic limiting potential was around −1.9 V (versus Ag/Ag+), and then its electrochemical window was about 3.9 V.| ILs | Cathodic limiting potential | Anodic limiting potential | Electrochemical window |
|---|---|---|---|
| V vs. Ag/Ag+ | V vs. Ag/Ag+ | V | |
| a Working electrode: glassy carbon; counter electrode: platinum wire; reference electrode: silver wire; scan rate: 10 mV s−1.b Ref. 36. | |||
| VEI-FSI | −1.5 | +1.8 | 3.3 |
| AMI-FSI | −1.9 | +2.0 | 3.9 |
| AEI-FSI | −1.8 | +2.0 | 3.8 |
| Im2o1-1-FSI | −2.1 | +1.9 | 4.0 |
| Im2o1-2-FSI | −1.8 | +1.9 | 3.7 |
| Im2o2-1-FSI | −2.1 | +2.1 | 4.2 |
| Im2o2-2-FSI | −1.8 | +2.1 | 3.9 |
| EMI-FSI | −2.2 | 1.9 | 4.1 |
| VEI-TFSI | −1.7 | 1.9 | 3.6 |
| AMI-TFSI | −2.0 | 2.1 | 4.1 |
| AEI-TFSI | −2.0 | 2.2 | 4.2 |
| Im2o1-1-TFSIb | −1.9 | 1.6 | 3.5 |
| Im2o1-2-TFSIb | −2.0 | 2.2 | 4.2 |
| Im2o2-1-TFSIb | −1.9 | 1.6 | 3.5 |
| Im2o2-2-TFSIb | −2.0 | 2.0 | 4.0 |
| EMI-TFSIb | −2.0 | 2.2 | 4.2 |
Generally, the electrochemical windows of TFSI-based pyrrolidinium, piperidinium, tetraalkylammonium and phosphonium ILs were over than 5 V, and it had been confirmed that the oxidation of TFSI anion and the reduction of their cations were responsible for the anodic and cathodic limits respectively.34 And the electrochemical stability of FSI-based pyrrolidinium, piperidinium and phosphonium ILs was close to the corresponding TFSI-based ILs,21,49 indicating that the oxidation stability of FSI anion was similar to that of TFSI anion. Nevertheless, for 1,3-dialkylimidazolium ILs, their cathodic and anodic limiting potentials were both governed by the cation, no matter whether the anion was TFSI or FSI.21,34 According to Table 2, introducing functional group into 1,3-dialkylimidazolium cation would somewhat affect the electrochemical stability. Compared with EMI cation, alkenyl- or ether-functionalization of cation could lead to narrower electrochemical windows. Especially the narrowest electrochemical windows appeared for VEI cation. This meant that the electrochemical stability of ether or alkenyl group was not as good as that of alkyl group, and vinyl group in VEI cation had higher electrochemical activity.
4. Conclusion
Several novel functionalized 1,3-dialkylimdazolium ILs based on FSI anion were synthesized and their physicochemical properties were investigated in detail. All of these ILs were liquids at ambient temperature owning low melting point. As expected, introduction of functionalized group and FSI anion collectively could help to obtain low-viscous and high-conductive ILs. The viscosities of these ILs were lower than 30 mPa s while their conductivities were also higher than 7 mS cm−1. AEI-FSI had the lowest viscosity (17.4 mPa s at 25 °C) which was the most outstanding one among all FSI-based ILs have been reported and it also had relatively higher conductivity (12.8 mS cm−1 at 25 °C). Most of these ILs had the electrochemical windows wider than 3.7 V which means that they were electrochemical stable enough to be new potential electrolytes for electrochemical fields.Acknowledgements
The authors would like to thank Research Center of Analysis and Measurement of Shanghai Jiao Tong University for their kind help in NMR tests. This work was financially supported by National Natural Science Foundation of China (Grants No. 21373136).References
- M. Armand, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621–629 CrossRef CAS PubMed.
- J. Dupont, R. F. De Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667–3692 CrossRef CAS PubMed.
- J. F. Brennecke and E. J. Maginn, AIChE J., 2001, 47, 2384–2389 CrossRef CAS.
- H. Zhao, S. Xia and P. Ma, J. Chem. Technol. Biotechnol., 2005, 80, 1089–1096 CrossRef CAS.
- R. Sheldon, Chem. Commun., 2001, 2399–2407 RSC.
- Z. Yang and W. Pan, Enzyme Microb. Technol., 2005, 37, 19–28 CrossRef CAS.
- M. Grätzel, J. Photochem. Photobiol., C, 2003, 4, 145–153 CrossRef.
- B. Li, L. Wang, B. Kang, P. Wang and Y. Qiu, Sol. Energy Mater. Sol. Cells, 2006, 90, 549–573 CrossRef CAS.
- R. Hagiwara and J. S. Lee, Electrochemistry, 2007, 75, 23–34 CrossRef CAS.
- M. J. A. Shiddiky and A. A. J. Torriero, Biosens. Bioelectron., 2011, 26, 1775–1787 CrossRef CAS PubMed.
- D. R. MacFarlane, N. Tachikawa, M. Forsyth, J. M. Pringle, P. C. Howlett, G. D. Elliott, J. H. Davis, M. Watanabe, P. Simon and C. A. Angell, Energy Environ. Sci., 2014, 7, 232–250 CAS.
- A. Lewandowski and A. Świderska-Mocek, J. Power Sources, 2009, 194, 601–609 CrossRef CAS.
- M. A. Navarra, MRS Bull., 2013, 38, 548–553 CrossRef CAS.
- S. Fang, L. Yang, J. Wang, H. Zhang, K. Tachibana and K. Kamijima, J. Power Sources, 2009, 191, 619–622 CrossRef CAS.
- R. Hagiwara, T. Hirashige, T. Tsuda and Y. Ito, J. Electrochem. Soc., 2002, 149, D1–D6 CrossRef CAS.
- J. Dupont, J. Braz. Chem. Soc., 2004, 15, 341–350 CrossRef CAS.
- V. Lockett, R. Sedev, J. Ralston, M. Horne and T. Rodopoulos, J. Phys. Chem. C, 2008, 112, 7486–7495 CAS.
- S. Zhang, N. Sun, X. He, X. Lu and X. Zhang, J. Phys. Chem. Ref. Data, 2006, 35, 1475–1517 CrossRef CAS.
- P. Bonĥte, A. P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Inorg. Chem., 1996, 35, 1168–1178 CrossRef.
- D. R. MacFarlane, S. A. Forsyth, J. Golding and G. B. Deacon, Green Chem., 2002, 4, 444–448 RSC.
- H. Matsumoto, H. Sakaebe, K. Tatsumi, M. Kikuta, E. Ishiko and M. Kono, J. Power Sources, 2006, 160, 1308–1313 CrossRef CAS.
- H. Sakaebe, H. Matsumoto and K. Tatsumi, J. Power Sources, 2005, 146, 693–697 CrossRef CAS.
- J. H. Davis Jr, Chem. Lett., 2004, 33, 1072–1077 CrossRef.
- K. Tsunashima, C. Nagai and M. Matsumiya, ECS Trans., 2013, 2013, 91–97 Search PubMed.
- H.-B. Han, J. Nie, K. Liu, W.-K. Li, W.-F. Feng, M. Armand, H. Matsumoto and Z.-B. Zhou, Electrochim. Acta, 2010, 55, 1221–1226 CrossRef CAS.
- J. S. Lee, N. D. Quan, J. M. Hwang, J. Y. Bae, H. Kim, B. W. Cho, H. S. Kim and H. Lee, Electrochem. Commun., 2006, 8, 460–464 CrossRef CAS.
- F. Mazille, Z. Fei, D. Kuang, D. Zhao, S. M. Zakeeruddin, M. Grätzel and P. J. Dyson, Inorg. Chem., 2006, 45, 1585–1590 CrossRef CAS PubMed.
- L. Zhao, J. I. Yamaki and M. Egashira, J. Power Sources, 2007, 174, 352–358 CrossRef CAS.
- Z. B. Zhou, H. Matsumoto and K. Tatsumi, Chem.–Eur. J., 2006, 12, 2196–2212 CrossRef CAS PubMed.
- Z. B. Zhou, H. Matsumoto and K. Tatsumi, Chem.–Eur. J., 2004, 10, 6581–6591 CrossRef CAS PubMed.
- Z. B. Zhou, H. Matsumoto and K. Tatsumi, Chem.–Eur. J., 2005, 11, 752–766 CrossRef CAS PubMed.
- K. Tsunashima and M. Sugiya, Electrochem. Commun., 2007, 9, 2353–2358 CrossRef CAS.
- S. Fang, Y. Tang, X. Tai, L. Yang, K. Tachibana and K. Kamijima, J. Power Sources, 2011, 196, 1433–1441 CrossRef CAS.
- H. Matsumoto, H. Sakaebe and K. Tatsumi, J. Power Sources, 2005, 146, 45–50 CrossRef CAS.
- W. A. Henderson, V. G. Young Jr, D. M. Fox, H. C. De Long and P. C. Trulove, Chem. Commun., 2006, 3708–3710 RSC.
- J. Zhang, S. Fang, L. Qu, Y. Jin, L. Yang and S.-I. Hirano, Ind. Eng. Chem. Res., 2014, 53, 16633–16643 CrossRef CAS.
- H.-B. Han, K. Liu, S.-W. Feng, S.-S. Zhou, W.-F. Feng, J. Nie, H. Li, X.-J. Huang, H. Matsumoto, M. Armand and Z.-B. Zhou, Electrochim. Acta, 2010, 55, 7134–7144 CrossRef CAS.
- H. Matsumoto, M. Yanagida, K. Tanimoto, M. Nomura, Y. Kitagawa and Y. Miyazaki, Chem. Lett., 2000, 922–923 CrossRef CAS.
- T. Mizumo, E. Marwanta, N. Matsumi and H. Ohno, Chem. Lett., 2004, 33, 1360–1361 CrossRef CAS.
- Z. Fei, D. Kuang, D. Zhao, C. Klein, H. A. Wee, S. M. Zakeeruddin, M. Grätzel and P. J. Dyson, Inorg. Chem., 2006, 45, 10407–10409 CrossRef CAS PubMed.
- G. H. Min, T. Yim, Y. L. Hyun, H. H. Dal, E. Lee, J. Mun, S. M. Oh and G. K. Young, Bull. Korean Chem. Soc., 2006, 27, 847–852 CrossRef CAS.
- T. Yim, Y. L. Hyun, H. J. Kim, J. Mun, S. Kim, S. M. Oh and G. K. Young, Bull. Korean Chem. Soc., 2007, 28, 1567–1572 CrossRef CAS.
- K. Tsunashima, Y. Ono and M. Sugiya, Electrochim. Acta, 2011, 56, 4351–4355 CrossRef CAS.
- D. Zhao, Z. Fei, H. A. Wee and P. J. Dyson, Int. J. Mol. Sci., 2007, 8, 304–315 CrossRef CAS.
- C. Liu, X. Ma, F. Xu, L. Zheng, H. Zhang, W. Feng, X. Huang, M. Armand, J. Nie, H. Chen and Z. Zhou, Electrochim. Acta, 2014, 149, 370–385 CrossRef CAS.
- N. Handa, T. Sugimoto, M. Yamagata, M. Kikuta, M. Kono and M. Ishikawa, J. Power Sources, 2008, 185, 1585–1588 CrossRef CAS.
- T. Makino, M. Kanakubo, T. Umecky, A. Suzuki, T. Nishida and J. Takano, J. Chem. Eng. Data, 2012, 57, 751–755 CrossRef CAS.
- K. Tsunashima, Y. Sakai and M. Matsumiya, Electrochem. Commun., 2014, 39, 30–33 CrossRef CAS.
- K. Tsunashima, A. Kawabata, M. Matsumiya, S. Kodama, R. Enomoto, M. Sugiya and Y. Kunugi, Electrochem. Commun., 2011, 13, 178–181 CrossRef CAS.
- M. Ishikawa, T. Sugimoto, M. Kikuta, E. Ishiko and M. Kono, J. Power Sources, 2006, 162, 658–662 CrossRef CAS.
- A. Guerfi, S. Duchesne, Y. Kobayashi, A. Vijh and K. Zaghib, J. Power Sources, 2008, 175, 866–873 CrossRef CAS.
- S. Seki, Y. Kobayashi, H. Miyashiro, Y. Ohno, Y. Mita, N. Terada, P. Charest, A. Guerfi and K. Zaghib, J. Phys. Chem. C, 2008, 112, 16708–16713 CAS.
- S. Tsuzuki, K. Hayamizu and S. Seki, J. Phys. Chem. B, 2010, 114, 16329–16336 CrossRef CAS PubMed.
- M. Kerner, N. Plylahan, J. Scheers and P. Johansson, Phys. Chem. Chem. Phys., 2015, 17, 19569–19581 RSC.
- H. Sumitaka, Method for producing N-alkyl-N′-alkylimidazolium salt, Japanese Patent, 2008088156, 2008.
- K. Tsunashima, S. Kodama, M. Sugiya and Y. Kunugi, Electrochim. Acta, 2010, 56, 762–766 CrossRef CAS.
- M. Kärnä, M. Lahtinen and J. Valkonen, J. Mol. Struct., 2009, 922, 64–76 CrossRef.
- M. Chai, Y. Jin, S. Fang, L. Yang, S.-I. Hirano and K. Tachibana, J. Power Sources, 2012, 216, 323–329 CrossRef CAS.
- R. Vijayaraghavan, M. Surianarayanan, V. Armel, D. R. MacFarlane and V. P. Sridhar, Chem. Commun., 2009, 6297–6299 RSC.
- H. B. Han, Y. X. Zhou, K. Liu, J. Nie, X. J. Huang, M. Armand and Z. B. Zhou, Chem. Lett., 2010, 39, 472–474 CrossRef CAS.
- N. Wongittharom, T.-C. Lee, I. M. Hung, S.-W. Lee, Y.-C. Wang and J.-K. Chang, J. Mater. Chem. A, 2014, 2, 3613 CAS.
- Y. Jin, S. Fang, M. Chai, L. Yang and S.-I. Hirano, Ind. Eng. Chem. Res., 2012, 51, 11011–11020 CrossRef CAS.
- R. Sescousse, K. A. Le, M. E. Ries and T. Budtova, J. Phys. Chem. B, 2010, 114, 7222–7228 CrossRef CAS PubMed.
- O. Borodin, W. Gorecki, G. D. Smith and M. Armand, J. Phys. Chem. B, 2010, 114, 6786–6798 CrossRef CAS PubMed.
- R. Ludwig and D. Paschek, ChemPhysChem, 2009, 10, 516–519 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12323f |
| This journal is © The Royal Society of Chemistry 2016 |