NO catalytic oxidation over an ultra-large surface area LaMnO3+δ perovskite synthesized by an acid-etching method†
Baohuai Zhaoa,
Rui Ran*a,
Li Suna,
Xingguo Guoa,
Xiaodong Wub and
Duan Weng*ab
aState Key Laboratory of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China. E-mail: ranr@tsinghua.edu.cn; duanweng@tsinghua.edu.cn
bKey Laboratory of Advanced Materials of Ministry of Education, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China
First published on 18th July 2016
Abstract
The perovskite oxides LaMnO3+δ were prepared by a citrate method and then etched by acid solution with different times. After etching, the perovskite structures of all the catalysts remained, the surface area enlarged apparently, and the NO catalytic oxidation activities were obviously promoted by increasing the etching time. Long-term experiments revealed the acceptable stability of the etched catalysts. The favorable activities of the etched LaMnO3+δ catalysts were mainly due to their high specific surface area, abundant activated oxygen species and vacancies, as well as the higher valence state of Mn ions resulting from the loss of La3+ in the perovskite structure.
Introduction
Mn-based perovskites are promising materials with good catalytic activities. They have been widely investigated for reactions involved in gas–solid phase heterogeneous catalysis such as CH4 combustion,1–4 CO oxidation,4–6 NO oxidation,2,5 and volatile organic compound oxidation,4,7–10 etc. For NO catalytic oxidation, Mn-based perovskites, especially LaMnO3+δ perovskites, have been mentioned a lot to substitute for commercial Pt-based catalysts because of their competitive oxidation activities over Pt-based catalysts.2,11,12LaMnO3+δ perovskite-type oxides are represented by the ideal general formula ABO3, where the larger cation La3+ has a dodecahedral coordination and the smaller Mn3+ cation has a six-fold coordination with O atoms.1–3,5 Perovskites are known as versatile materials which allow the incorporation of different cations in their structures.13 Many earlier studies had focused on the substitution of A or B cations with elements of different sizes and valences to introduce structural defects (anionic or cationic vacancies) in Mn-based perovskites.3–5,8,11–17 In the literatures,11,14,15 the influences of nonstoichiometry on the catalytic performance of Mn-based perovskites for NO oxidation by adjusting the A or B sites were well investigated; and their activities were optimized. And most of all, Kim et al.12 reported that the Sr-doped Mn-based perovskite catalysts showed NO oxidation activities similar to or higher than those of Pt-based catalysts under realistic conditions. On the other hand, among the parameters affecting the catalytic activities of LaMnO3+δ catalysts, specific surface area and redox properties also appear to be critical.5,13,18 Etching method using acid solution is one of the effective methods to increase the specific surface area, change the valence states of active ions and selectively remove the inert species from the structure to create more active sites.19–21 Recently, Si and co-workers18 used diluted HNO3 to selectively remove La3+ cations from the LaMnO3+δ perovskite and obtained a novel γ-MnO2-like material. Such a material showed really higher activity for CO oxidation than the normal LaMnO3+δ. It provided a new methodological route to design high-performance oxidation catalysts. However, γ-MnO2 is a kind of oxide owning a lot of discontinuities and structural faults. It is very easy to transform to some other kind of MnOx structures.22,23 Considering the demand of thermal stability for a typical NO oxidation catalyst, it is worth optimizing the parameters of the etching method, in order to maintain materials in the more stable structures.
In the present study, a series of LaMnO3+δ perovskite catalysts were synthesized using citrate method and etched by acid solution with different time. Then we investigated the evolution of structures, morphologies and physicochemical properties, as well as the NO catalytic oxidation activity of the catalysts. The results helped to understand the correlations between catalytic performance and structural properties of these catalysts.
Experimental
The LaMnO3+δ were prepared by citrate method which was similar to those described in literatures.1,11,15 The stoichiometric metallic nitrates of Mn(NO3)2 (50% solution, Aladdin, China) and La(NO3)3·6H2O (99.9%, Aladdin, China) were dissolved in deionized water. Then 10% excess citric acid over the number of ionic equivalents of cations was added to the aqueous solution. The resulting solution was stirred and evaporated at 90 °C until the formation of a gel. The gel was dried in an oven at 110 °C overnight, forming a spongy amorphous citrate precursor. The precursor was milled and calcined with a heating rate of 1 °C min−1 in static air at 300 °C for 1 h to decompose the organics, and then at 750 °C for 5 h to obtain the LaMnO3+δ perovskite structure. For the acid etching process, LaMnO3+δ was added into a HNO3 solution of 1 mol L−1 with magnetic stirring for 1 h, 5 h, and 12 h. The products were washed for several times until the filtrated water was neutral, and then dried at 110 °C overnight. Finally, the dried samples were calcined at 300 °C for 3 h with a heating rate of 10 °C min−1 in static air. For a good comparison, LaMnO3+δ without etching process was also washed, dried and calcined in the same conditions. The catalysts were denoted as LMO, LMO-1, LMO-5 and LMO-12, respectively, and the numbers here were corresponded to the etching time.The catalysts were characterized by X-ray diffraction (XRD; D8 Advance, Bruker, Germany), using Cu Kα radiation (λ = 0.15418 nm); the X-ray tube was operated at 40 kV and 300 mA. Powder XRD patterns were recorded at 0.02° intervals in the range 10° ≤ 2θ ≤ 70°, at a scanning rate of 6° min−1. Morphologies of the catalysts were measured using scanning electron microscopy (SEM; JSM-7001F, JEOL) at an accelerating voltage of 30 kV. Brunauer–Emmett–Teller (BET) specific areas were determined based on N2 adsorption/desorption recorded at −196 °C using a JW-BK122F instrument (Beijing JWGB, China). Prior to N2 adsorption, the catalysts were outgassed at 220 °C for 2 h to desorb moisture adsorbed on the surfaces and inside the porous networks. The X-ray photoelectron spectroscopy (XPS) analysis was performed on a PHI-5300 ESCA system equipped with a Al Kα radiation source under UHV (1.33 × 10−8 Pa) conditions. Before the XPS tests the catalysts were pre-treated at 300 °C in 10% O2/N2 (50 mL min−1) for 30 min. The binding energies (B.E.) of the elements were calibrated internally by the carbon deposit C 1s binding energy at 284.8 eV. H2 temperature-programmed reduction (H2-TPR) was performed using a Micromeritics Auto Chem II 2920 instrument. For each experiment, the catalyst (approximately 50 mg) was placed in a U-shaped quartz tube and pre-treated at 300 °C in 10% O2/N2 (50 mL min−1) for 30 min. Then the temperature was ramped down to 0 °C, and He was introduced to purge the residual oxygen. The catalysts were reduced in a flow of 10% H2/Ar (50 mL min−1) at 0 to 1000 °C; the signal was recorded using a thermal conductivity detector. The reduction curves were deconvoluted into sub-peaks by Peakfit v4.12 software. H2 consumption for each peak was determined quantitatively based on TPR calibration runs using a standard silver oxide sample (Micromeritics) in place of the catalyst.
The NO catalytic oxidation was performed in a fixed-bed quartz reactor (i.d. 12 mm) at various temperatures from room temperature to 500 °C. Typically, a well-distributed mixture of catalyst (100 mg) and quartz sand (300 mg) was placed in the reactor. Gases (500 ppm NO, 10% O2 balanced with N2) were mixed well before fed into the reactor system; the total flow rate of the reactant gas mixture was kept at 500 mL min−1, corresponding to a gas hourly space velocity (GHSV) of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. Before each catalytic test, the catalyst was flushed with 10% O2/N2 (500 mL min−1) at 300 °C for 30 min to remove the adsorbed species from the surface and then cooled down to room temperature. A Fourier-transform infrared spectrometer (MKS Multigas™ 2030 gas-phase) with a spectral resolution of 0.5 cm−1 was used to quantify the outlet gas concentrations. No other nitrogen-containing oxides except for NO and NO2 were detected. Thus the NO conversion ([NO2]outlet/([NO2]outlet + [NO]outlet) × 100%) was calculated with the temperature kept stable for 20 min. For the long-term reactions, temperature was set at 250 °C
000 h−1. Before each catalytic test, the catalyst was flushed with 10% O2/N2 (500 mL min−1) at 300 °C for 30 min to remove the adsorbed species from the surface and then cooled down to room temperature. A Fourier-transform infrared spectrometer (MKS Multigas™ 2030 gas-phase) with a spectral resolution of 0.5 cm−1 was used to quantify the outlet gas concentrations. No other nitrogen-containing oxides except for NO and NO2 were detected. Thus the NO conversion ([NO2]outlet/([NO2]outlet + [NO]outlet) × 100%) was calculated with the temperature kept stable for 20 min. For the long-term reactions, temperature was set at 250 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24,25 and the activities were continuously evaluated for about 20 h, using the same equipment and test conditions mentioned above.
24,25 and the activities were continuously evaluated for about 20 h, using the same equipment and test conditions mentioned above.
Results and discussion
Structures and morphologies of LaMnO3+δ
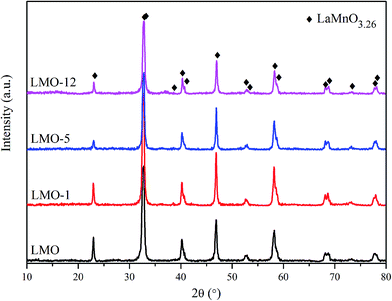
The XRD patterns of the catalysts are shown in Fig. 1. All of the patterns can be assigned to LaMnO3.26 structure (JCPDS #50-0299, rhombohedral, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c)26 without any impurity diffraction peaks, indicating that single-phase perovskite were obtained using the current synthetic method. The diffraction peaks of the four catalysts appear at the same positions. It demonstrates no obvious change of the phase structures even after etching for 1–12 h. However, the diffraction peaks of LMO-5 and LMO-12 show lower intensities, which corresponds to their poor crystallinity compared to LMO and LMO-1. On the other hand, there is a possibility that MnOx existing in the etched perovskite catalysts but with amorphous feature on the surface of the perovskite. It would not be easily detected by the XRD measurement.
c)26 without any impurity diffraction peaks, indicating that single-phase perovskite were obtained using the current synthetic method. The diffraction peaks of the four catalysts appear at the same positions. It demonstrates no obvious change of the phase structures even after etching for 1–12 h. However, the diffraction peaks of LMO-5 and LMO-12 show lower intensities, which corresponds to their poor crystallinity compared to LMO and LMO-1. On the other hand, there is a possibility that MnOx existing in the etched perovskite catalysts but with amorphous feature on the surface of the perovskite. It would not be easily detected by the XRD measurement.
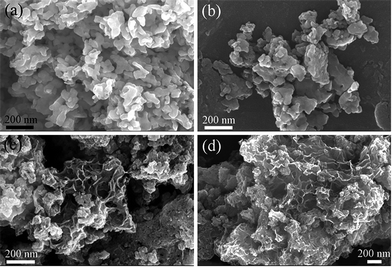
Fig. 2 shows SEM images of the catalysts. In Fig. 2(a) and (b), LMO and LMO-1 present the similar morphologies with those severely sintered and aggregative particles. In contrast, the morphologies of LMO-5 and LMO-12 in Fig. 2(c) and (d) display as many particles with “sheets-like” margins and porous structures. Chemical elementary analyses were realized by EDS to estimate the chemical composition of the catalysts and the results are shown in Table 1. The decreasing trend of La content is observed in the order of LMO > LMO-1 > LMO-5 > LMO-12, which confirms the successful etching effect by HNO3 solution. With the remove of La3+ in A sites, oxygen vacancies can be produced and the valence state of Mn can also be improved due to the electric neutrality principle. Meanwhile, the specific surface areas summarized in Table 1 are found to be increasing with extending the etching time. Of the four catalysts, LMO-12 has the largest specific surface area of 126.2 m2 g−1, which is superior to that of LMO (16.3 m2 g−1) as well as those of the typical Mn-based perovskites synthesized by similar method in other literatures (less than 30 m2 g−1).1,11,14,15,27,28 The ultra-large surface area reveals that the acid etching method is effective on improving the specific surface area of the perovskites.
| Catalyst | SBET (m2 g−1) | Atom. (%) by EDS | Atom. (%) by XPS | H2 consumption (mmol g−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| La | Mn | O | La | Mn | O | α | β | γ | δ | ||
| LMO | 16.3 | 15.0 | 15.2 | 70.8 | 17.1 | 17.8 | 65.2 | 0.17 | 0.38 | 1.09 | 1.32 |
| LMO-1 | 36.4 | 13.0 | 15.5 | 71.5 | 10.1 | 24.8 | 65.1 | 0.25 | 0.48 | 1.82 | 1.31 |
| LMO-5 | 74.2 | 6.5 | 21.0 | 72.6 | 5.4 | 31.0 | 63.7 | 0.40 | 1.23 | 2.12 | 1.16 |
| LMO-12 | 126.2 | 5.4 | 27.7 | 66.9 | 3.1 | 33.8 | 63.1 | 0.49 | 1.71 | 2.44 | 0.91 |
Surface properties
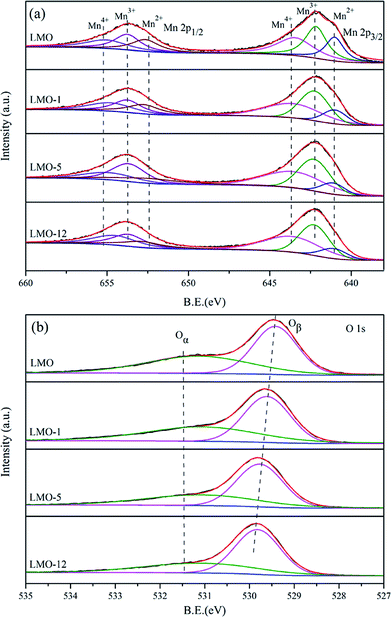
As shown in Table 1, the La surface atomic percentages of LMO, LMO-1, LMO-5 and LMO-12 tested by XPS are 17.1%, 10.1%, 5.4% and 3.1%, respectively. The trend of La content is consistent with the EDS results, which further confirms the loss of La during the etching process. Fig. 3 shows the deconvoluted XPS spectra of Mn 2p, and O 1s of the four catalysts. Two main peaks due to Mn 2p3/2 and Mn 2p1/2 are shown in Fig. 3(a). Each of them can be well fitted into three sub-peaks at 643–644 eV, 641–642 eV, and 640–641 eV which are assigned to the 2p3/2 peak of Mn4+, Mn3+, Mn2+, respectively.1–3,15,26 The relative atomic percentages of Mn4+, Mn3+, Mn2+ on the surface of the catalysts can be estimated from the relative area of the sub-peaks, and the integrated results are displayed in Table 2. The Mn4+/Mnn+ ratio is apparently increasing with the increase of etching time. With the removing of La3+ ions, partial Mn3+ will be oxidized to Mn4+ to maintain the charge balance of the surface of the perovskites. In Fig. 3(b), the O 1s XPS spectra are fitted into two sub-peaks which represent two surface oxygen species. The higher binding energy peak can be ascribed to the surface chemisorbed oxygen such as defect oxides and oxygen ions with low coordination (Oα), and the one with lower binding energy is assigned to the lattice oxygen (Oβ).1–3 The B.E. of Oα, shows almost no difference in each catalyst, while the B.E. of Oβ has an increasing trend with increasing etching time. Higher B.E. of Oβ means that the lattice oxygen atoms are less closely bonded in the structures, which probably results in stronger mobility of the oxygen species.| Catalyst | Mn 2p3/2 | O 1s | ||||||
|---|---|---|---|---|---|---|---|---|
| Mn4+ | Mn3+ | Mn2+ | Oα | Oβ | ||||
| B.E. (eV) | Mn4+/Mnn+ (%) | B.E. (eV) | Mn3+/Mnn+ (%) | B.E. (eV) | Mn2+/Mnn+ (%) | B.E. (eV) | B.E. (eV) | |
| LMO | 643.5 | 38.4 | 642.2 | 38.2 | 640.9 | 23.4 | 531.1 | 529.4 |
| LMO-1 | 643.5 | 43.1 | 642.2 | 41.1 | 640.9 | 15.8 | 531.1 | 529.6 |
| LMO-5 | 643.5 | 45.7 | 642.2 | 42.8 | 641.1 | 11.6 | 531.1 | 529.8 |
| LMO-12 | 643.6 | 46.1 | 642.2 | 41.8 | 641.1 | 12.2 | 531.1 | 529.9 |
Reducibility
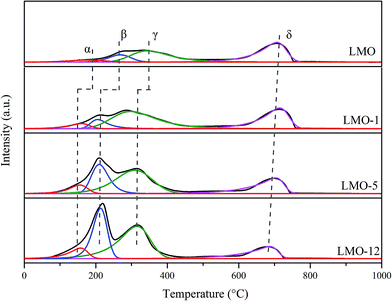
The reducibility of the catalysts was investigated using H2-TPR experiments; the results are shown in Fig. 4. All these profiles display two obvious reduction regions: 50–500 °C and 500–800 °C. The former region can be divided into three reduction peaks (α, β and γ), while the later one contains one reduction peak (δ). According to the results reported previously, peak α was assigned to the removal of the surface and most readily oxygen species.2–4,29 Peak β was mainly led by the reduction of Mn4+ to Mn3+.2,3,11,15 Peak γ was attributed to the reduction of Mn3+ ions located in coordination-unsaturated microenvironments to Mn2+ before 500 °C.4,29 Peak δ was associated with the reduction of the rest Mn3+ in the bulk to Mn2+.2,3 It is found that the former three reduction peaks of the etched catalysts shift to lower temperature obviously compared to LMO, suggesting their superior oxygen mobility. Quantitative analyses on these reduction peaks are carried out, and the H2 consumption of each peak is summarized in Table 1. It is observed that the H2 consumptions corresponding to former three reduction peaks increased simultaneously when extending the etching time. On the contrary, H2 consumptions corresponding to peak δ decrease in the sequence of LMO, LMO-1, LMO-5 and LMO-12. These results demonstrate that the etching process not only dramatically creates more active oxygen but also increase the valence state of the Mn ions, both of which are highly beneficial for catalytic oxidation activities.30Catalytic activities for NO oxidation
The NO conversion as a function of temperature for the four catalysts was determined; the results are shown in Fig. 5(a). As expected, the reaction is kinetically limited at low temperatures and becomes thermodynamically controlled at high temperatures.31–34 Among the four catalysts, LMO-12 and LMO-5 show superior activities compared to LMO and LMO-1. They present the similar activities in the whole test temperature range and own the maximum NO conversion of 86% at 290 °C. In contrast, the maximum NO conversions of LMO and LMO-1 are 79% at 300 °C and 69% at 315 °C, respectively. Fig. 5(b) shows the results of long-term, i.e., 20 h, activity tests at 250 °C. All the catalysts show acceptable stability during the long-term reactions, which is probably related to the stable structural feature of perovskites. The NO conversions of LMO, LMO-1, LMO-5, and LMO-12 decrease by ∼6%, ∼6%, ∼4%, and ∼5%, respectively. It is indicated that the acid etching process may be a promising optimizing method for perovskite-type catalytic materials.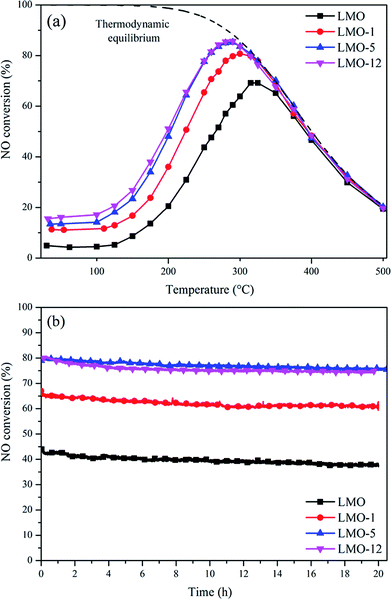 | ||
Fig. 5 (a) NO conversions at various temperatures and (b) long term reaction activities at 250 °C over the four catalysts. Reaction conditions: 500 ppm NO, 10% O2, and N2 balance; GHSV = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. 000 h−1. | ||
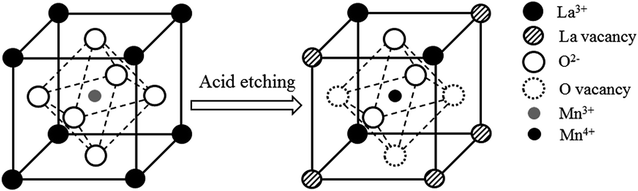
In LaMnO3+δ perovskites, La sites are the inert components for NO oxidation reaction. When La3+ is partially removed from the lattice of LaMnO3+δ perovskite, abundant vacancies appear in A sites of the perovskite lattice, leading to a loose structural framework which may contribute the extra surface area. The charge compensation follows through the formation of oxygen vacancies which is in equilibrium with the formation of Mn4+. According to this, the scheme of the effects of acid etching on LaMnO3+δ perovskite is established in Fig. 6. It reveals that both oxygen vacancies and Mn4+ ions can be created during the etching process due to the charge compensation, and the lattice oxygen species are more weakly bonded. All these changes have been confirmed by the above XPS and TPR results of LMO, LMO-1, LMO-5 and LMO-12. On the basis of some literatures on NO oxidation catalysis, nitrates were important intermediates in the oxidation process.35,36 We used LMO-12 to perform the in situ FTIR experiment (Fig. S1†) and confirmed that nitrates were main intermediates during the adsorption process. The NO oxidation over the catalysts would therefore follow the nitrates decomposition pathways. The oxygen vacancies and Mn4+ ions could provide most of the active sites for adsorption and activation of NO, respectively, and active oxygen species in perovskite structure were responsible for the generation of nitrates. Consequently, those LaMnO3+δ perovskites etched by acid solution in the present work showed superior catalytic activities, which possessed all the advantage parameters including ultra-large surface area, abundant vacancies, higher valence state of Mn ions and more active oxygen species. In addition, owing to the maintenance of the perovskite structure, all the four catalysts exhibited favorable reaction stability in long-term catalytic tests.
Conclusions
The NO oxidation activities of LaMnO3+δ perovskite etched by acid solution were studied. The La atoms were successfully removed from the lattice by etching method while the perovskite structures were still remained. Increasing etching time had significant effect on structural properties and the NO catalytic oxidation activities. Among the catalysts investigated, the LaMnO3+δ etched for 5 h and 12 h showed ultra-large surface area of >100 m2 g−1, and of course the superior activities with a maximum NO conversion of 86% at 290 °C. Oxygen vacancies, higher valence state of Mn ions, as well as the active oxygen species generated during the etching process were all responsible for the activities. It was also worth to be mentioned that the etched LaMnO3+δ catalysts could maintain as good stability as the original LaMnO3+δ in the long-term reaction, owing to their structural stability. It is permitted the acid etching process to be a promising optimizing method for perovskite-type catalytic materials in this filed.Acknowledgements
The authors acknowledge financial support from the National Key Research and Development Program of China (Project 2016YFC0205000) and the Ministry of Science and Technology of China (Project 2015AA034603). We also thank the Key Laboratory of Advanced Materials (MOE) of School of Materials Science and Engineering for performing material characterizations.Notes and references
- E. Arendt, A. Maione, A. Klisinska, O. Sanz, M. Montes, S. Suarez, J. Blanco and P. Ruiz, Appl. Catal., A, 2008, 339, 1–14 CrossRef CAS.
- E. Lim, Y. J. Kim, J. H. Kim, T. Ryu, S. Lee, B. K. Cho, I.-S. Nam, J. W. Choung and S. Yoo, J. Catal., 2014, 319, 182–193 CrossRef.
- S. Ponce, M. Pena and J. Fierro, Appl. Catal., B, 2000, 24, 193–205 CrossRef CAS.
- S. Liang, F. Teng, G. Bulgan and Y. Zhu, J. Phys. Chem. C, 2007, 111, 16742–16749 CAS.
- H. Zhu, P. Zhang and S. Dai, ACS Catal., 2015, 5, 6370–6385 CrossRef CAS.
- K.-S. Song, S.-K. Kang and S. D. Kim, Catal. Lett., 1997, 49, 65–68 CrossRef CAS.
- J.-M. Giraudon, A. Elhachimi, F. Wyrwalski, S. Siffert, A. Aboukais, J.-F. Lamonier and G. Leclercq, Appl. Catal., B, 2007, 75, 157–166 CrossRef CAS.
- C. Zhang, W. Hua, C. Wang, Y. Guo, Y. Guo, G. Lu, A. Baylet and A. Giroir-Fendler, Appl. Catal., B, 2013, 134, 310–315 CrossRef.
- G. Liu, J. Li, K. Yang, W. Tang, H. Liu, J. Yang, R. Yue and Y. Chen, Particuology, 2015, 19, 60–68 CrossRef CAS.
- C. Zhang, W. Hua, C. Wang, Y. Guo, Y. Guo, G. Lu, A. Baylet and A. Giroir-Fendler, Appl. Catal., B, 2013, 134, 310–315 CrossRef.
- J. Wang, Y. Su, X. Wang, J. Chen, Z. Zhao and M. Shen, Catal. Commun., 2012, 25, 106–109 CrossRef CAS.
- C. H. Kim, G. Qi, K. Dahlberg and W. Li, Science, 2010, 327, 1624–1627 CrossRef CAS PubMed.
- J. Zhu and A. Thomas, Appl. Catal., B, 2009, 92, 225–233 CrossRef CAS.
- M. Shen, Z. Zhao, J. Chen, Y. Sun, J. Wang and X. Wang, J. Rare Earths, 2013, 31, 119–123 CrossRef CAS.
- J. Chen, M. Shen, X. Wang, G. Qi, J. Wang and W. Li, Appl. Catal., B, 2013, 134, 251–257 CrossRef.
- A. Glisenti, M. Pacella, M. Guiotto, M. Natile and P. Canu, Appl. Catal., B, 2016, 180, 94–105 CrossRef CAS.
- M. Alifanti, J. Kirchnerova and B. Delmon, Appl. Catal., A, 2003, 245, 231–244 CrossRef CAS.
- W. Si, Y. Wang, Y. Peng and J. Li, Angew. Chem., Int. Ed., 2015, 54, 7954–7957 CrossRef CAS PubMed.
- J. Quiroz, J.-M. Giraudon, A. Gervasini, C. Dujardin, C. Lancelot, M. Trentesaux and J.-F. Lamonier, ACS Catal., 2015, 5, 2260–2269 CrossRef CAS.
- M. E. Grass, Y. Yue, S. E. Habas, R. M. Rioux, C. I. Teall, P. Yang and G. A. Somorjai, J. Phys. Chem. C, 2008, 112, 4797–4804 CAS.
- K. Tsujino and M. Matsumura, Electrochim. Acta, 2007, 53, 28–34 CrossRef CAS.
- F. T. Shuhui Liang, G. Bulgan, R. Zong and Y. Zhu, J. Phys. Chem. C, 2008, 112, 5307–5315 Search PubMed.
- J. H. Albering, in Handbook of Battery Materials, ed. C. Daniel and J. O. Besenhard, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2nd edn, 2011, pp. 87–123 Search PubMed.
- Z. Wu, N. Tang, L. Xiao, Y. Liu and H. Wang, J. Colloid Interface Sci., 2010, 352, 143–148 CrossRef CAS PubMed.
- N. Tang, Y. Liu, H. Wang and Z. Wu, J. Phys. Chem. C, 2011, 115, 8214–8220 CAS.
- C. Zhang, C. Wang, W. Zhan, Y. Guo, Y. Guo, G. Lu, A. Baylet and A. Giroir-Fendler, Appl. Catal., B, 2013, 129, 509–516 CrossRef CAS.
- O. P. Taran, A. B. Ayusheev, O. L. Ogorodnikova, I. P. Prosvirin, L. A. Isupova and V. N. Parmon, Appl. Catal., B, 2016, 180, 86–93 CrossRef CAS.
- H. Xu, Z. Qu, C. Zong, F. Quan, J. Mei and N. Yan, Appl. Catal., B, 2016, 186, 30–40 CrossRef CAS.
- Y. Liu, H. Dai, J. Deng, Y. Du, X. Li, Z. Zhao, Y. Wang, B. Gao, H. Yang and G. Guo, Appl. Catal., B, 2013, 140–141, 493–505 CrossRef CAS.
- H. Lu, P. Zhang, Z.-A. Qiao, J. Zhang, H. Zhu, J. Chen, Y. Chen and S. Dai, Chem. Commun., 2015, 51, 5910–5913 RSC.
- I. Atribak, I. Such-Basáñez, A. Bueno-López and A. García, J. Catal., 2007, 250, 75–84 CrossRef CAS.
- P. S. Metkar, V. Balakotaiah and M. P. Harold, Catal. Today, 2012, 184, 115–128 CrossRef CAS.
- B. Azambre, I. Atribak, A. n. Bueno-López and A. García-García, J. Phys. Chem. C, 2010, 114, 13300–13312 CAS.
- B. Zhao, R. Ran, X. Wu and D. Weng, Appl. Catal., A, 2016, 514, 24–34 CrossRef CAS.
- W. Wang, N. Kapur, G. Yuan, B. Shan, M. Nguyen, U. M. Graham, B. H. Davis, G. Jacobs, K. Cho and X. Hao, Science, 2012, 337, 832–835 CrossRef CAS PubMed.
- B. Shen, X. Lin and Y. Zhao, Chem. Eng. J., 2013, 222, 9–15 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12308b |
| This journal is © The Royal Society of Chemistry 2016 |