Retracted Article: Experimental and theoretical studies of the nanostructured {Fe3O4@SiO2@(CH2)3Im}C(CN)3 catalyst for 2-amino-3-cyanopyridine preparation via an anomeric based oxidation†
Mohammad Ali
Zolfigol
*a,
Mahya
Kiafar
a,
Meysam
Yarie
a,
Avat(Arman)
Taherpour
bc and
Mahdi
Saeidi-Rad
a
aDepartment of Organic Chemistry, Faculty of Chemistry, Bu-Ali Sina University, Hamedan, Iran. E-mail: zolfi@basu.ac.ir; mzolfigol@yahoo.com; Fax: +98 8138257407; Tel: +98 8138282807
bDepartment of Organic Chemistry, Razi University, P.O. Box: 67149-67346, Kermanshah, Iran
cMedical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
First published on 17th May 2016
Abstract
2-Amino-4,6-diphenylnicotinonitriles were prepared by using Fe3O4@SiO2@(CH2)3Im}C(CN)3 as a nanostructured catalyst with an ionic liquid tag under solvent free and benign conditions. Experimental evidence and theoretical studies confirmed that the final step of the synthetic pathway proceeded via an anomeric based oxidation mechanism. A good range of aromatic aldehydes were condensed with acetophenone derivatives, malononitrile and ammonium acetate to afford their corresponding products in short reaction times and good to high yields.
Introduction
Knowledge based development of one-pot multi-component reactions (MCRs) over routine multi-step synthesis pathways, has advantages such as high atom economy, instantaneous access to large molecules with diverse functionality, improvement of the synthetic efficacy and simplicity of the formation of complex compounds, avoiding time consuming and costly purification processes.1–3 Thus, designing of novel one-pot MCRs for different purposes has attracted great attention from synthetic organic chemists.Among the pyridine ring systems which present varied chemotherapeutic and pharmacological applicabilities,4–6 2-amino-3-cyanopyridines raised substantial medicinal and synthetic attention since this widespread heterocyclic molecule allowed access to many demonstrated bio-active species such as IKK-b inhibitors,7 A2A adenosine receptor antagonist,8 and potent inhibitor of HIV-1 integrase.9
Hence, in consequence of the aforementioned biological significances of these versatile structural motifs, a number of procedures have been reported on this topic like microwave or ultrasound irradiation,10–13 hexadecyldimethyl benzyl ammonium bromide and triethylamine,14 DMF,15 acetic acid,16 Fe3O4,17 cellulose–SO3H18 and using earth Lewis acid catalyst.19 Although, several catalyst and protocol have been studied for the preparation of the 2-amino-3-cyanopyridine derivatives, the methods suffer from one or more imperfection such as toxic solvent like benzene,20 multi-steps reaction pathway,21 high temperature and microwave assistance,22,23 prolonged reaction times and harsh reaction conditions with low yields. In the light of these facts, surveying a straightforward and newer recyclable catalytic environmentally congruous approach for the synthesis of these compounds is still a great day required and an interesting mission to the methodologists and would be valuable.
In this, to conquer to the above mentioned hardships and in order to continue our work on the maturation of the design, building, applications and knowledge-based promotion of solid acids,24 inorganic acidic salts,25 nano-sphere silica with a tag of ionic liquid,26 new nanostructured ionic liquids, molten salts27 and N-halo reagent28 for organic functional group interconversion, we would like to inspect the application of {Fe3O4@SiO2@(CH2)3Im}C(CN)3 as a magnetically separable and reutilizable heterogeneous catalyst at the preparation of the 2-amino-3-cyanopyridines under solvent-free and mild reaction conditions as painted in Scheme 1.
 | ||
| Scheme 1 Catalytic applicability of the {Fe3O4@SiO2@(CH2)3Im}C(CN)3 in one-pot synthesis of 2-amino-3-cyanopyridines. | ||
Results and discussion
The silica-coated magnetic nanoparticles with a tag of ionic liquid as a heterogeneous catalyst was prepared according to our previously reported literature as presented in Scheme 2.29First of all, to find the best catalyst for the synthesis of 2-amino-3-cyanopyridine derivatives, several catalysts, including solid acids, organic and inorganic acids, different metal based inorganic salts and also the un-catalyzed condition were explored upon the reaction between benzaldehyde, acetophenone, malononitrile and ammonium acetate as a nitrogen source under solvent-free conditions at 100 °C and the achieved data were embedded in Table 1. From the obtained data in Table 1, it can be inferred that in addition to the {Fe3O4@SiO2@(CH2)3Im}C(CN)3 (entry 4), HIO3 (entry 3) and [Dsim]Cl (entry 7), could act as promoter for the model reaction in comparatively short times with good yields, but from the recovery and reusability viewpoint the {Fe3O4@SiO2@(CH2)3Im}C(CN)3 is the best choice and therefore, it is selected as a magnetically separable and reutilizable heterogeneous catalyst for the preparation of the 2-amino-3-cyanopyridines under solvent-free and mild reaction conditions.
| Entry | Used catalyst | Load of catalyst | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), acetophenone (1 mmol), malononitrile (1 mmol) and ammonium acetate (3 mmol). b Isolate yields. c Hydroxylamine-O-sulfonic acid. d 1-Carboxymethyl pyridinium chloride. e 1,3-Disulfonic acid imidazolium chloride. | ||||
| 1 | — | — | 150 | 60 |
| 2 | HOSAc | 20 mol% | 150 | 60 |
| 3 | HIO3 | 10 mol% | 40 | 88 |
| 4 | {Fe3O4@SiO2@(CH2)3Im}C(CN)3 | 7 mg | 40 | 86 |
| 5 | [cmpy]Cld | 10 mol% | 40 | 80 |
| 6 | Silica sulfuric acid | 10 mg | 50 | 70 |
| 7 | [Dsim]Cle | 10 mol% | 35 | 90 |
| 8 | Nano carbamoylsulfamic acid | 10 mol% | 40 | 75 |
| 9 | Nano 2-carbamoylhydrazine-1-sulfonic acid | 10 mol% | 50 | 70 |
| 10 | ZrOCl2·8H2O | 10 mol% | 50 | 77 |
| 11 | Al(HSO4)3 | 10 mol% | 60 | 68 |
| 12 | Bi(NO3) | 10 mol% | 60 | 73 |
| 13 | Ni(NO3)2·6H2O | 10 mol% | 70 | 60 |
| 14 | Mg(NO3)2·6H2O | 10 mol% | 75 | 81 |
| 15 | Zn(NO3)2·6H2O | 10 mol% | 65 | 78 |
| 16 | NH4NO3 | 10 mol% | 75 | 83 |
| 17 | FeCl3 | 10 mol% | 80 | 82 |
| 18 | HNO3 | 10 mol% | 90 | 30 |
| 19 | H3PO4 | 10 mol% | 90 | 61 |
Afterward, to find the best experimental reaction conditions for the synthesis of 2-amino-3-cyanopyridine derivatives, the reaction of the benzaldehyde, acetophenone, malononitrile and ammonium acetate was picked out as a test reaction and the different amounts of the magnetically separable heterogeneous catalyst, different temperatures and solvents were investigated. The attained data are described in Table 2. The obtained data have shown that the optimized conditions for the model reaction is where, when the reaction was accomplished using acatalytic amount of magnetically core–shell catalyst under solvent-free conditions at 100 °C (Table 2, entry 6).
| Entry | Solvent | Load of catalyst (mg) | Temperature (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), acetophenone (1 mmol), malononitrile (1 mmol) and ammonium acetate (3 mmol). b Isolate yields. | |||||
| 1 | — | 10 | 80 | 60 | 80 |
| 2 | — | 10 | 100 | 40 | 85 |
| 3 | — | 10 | 115 | 65 | 83 |
| 4 | — | — | 100 | 150 | 60 |
| 5 | — | 5 | 100 | 40 | 82 |
| 6 | — | 7 | 100 | 40 | 86 |
| 7 | EtOH | 7 | Reflux | 90 | 25 |
| 8 | H2O | 7 | Reflux | 90 | 25 |
| 9 | CH2Cl2 | 7 | Reflux | 90 | Trace |
| 10 | EtOAc | 7 | Reflux | 90 | Trace |
| 11 | n-Hexan | 7 | Reflux | 90 | 35 |
| 12 | CH3CN | 7 | Reflux | 95 | 30 |
After evaluation of data for the optimization of the reaction conditions for the synthesis of 2-amino-3-cyanopyridine derivatives, to confirm the capability and the efficacy of the designed method to the 2-amino-3-cyanopyridines, a agreeable extent of aromatic aldehydes (bearing electron-withdrawing and electron-releasing groups and halogens) were subjected to the reaction with acetophenones (including acetophenone, 4-methoxy acetophenone and 4-hydroxy acetophenone), malononitrile and ammonium acetate under the optimized reaction conditions to provide the related desired adducts in relatively short periods of time with good to high yields as embedded in Table 3.
| Entry | X | Y | Product | Time (min) | Yieldb (%) | Mp (°C)/Lit. Mp [ref.] |
|---|---|---|---|---|---|---|
| a Reaction conditions: arylbenzaldehyde (1 mmol), acetophenone derivatives (1 mmol), malononitrile (1 mmol) and ammonium acetate (3 mmol). b Isolate yields. | ||||||
| 1 | H | H | 1a | 40 | 86 | 187–188/186–187 [17] |
| 2 | 4-Cl | H | 1b | 25 | 88 | 234–236/233–235 [17] |
| 3 | 4-OMe | H | 1c | 40 | 80 | 174–176/181–182 [17] |
| 4 | 2-Cl | H | 1d | 50 | 80 | 194–197/193–196 [33b] |
| 5 | 4-NO2 | H | 1e | 55 | 81 | 223–225/212–213 [17] |
| 6 | 4-F | H | 1f | 30 | 90 | 148–150/— [31] |
| 7 | 4-Br | H | 1g | 35 | 89 | 195–197/225–228 [33a] |
| 8 | 4-Me | H | 1h | 45 | 82 | 231–233/— [33a] |
| 9 | 3-NO2 | H | 1i | 30 | 87 | 200–202/— [19] |
| 10 | H | 4-OH | 1j | 40 | 80 | 233–235/— [33a] |
| 11 | 4-CN | H | 1k | 30 | 88 | 184–186/— [30] |
| 12 | 4-Cl | 4-OH | 1l | 45 | 80 | 270–272/— [33a] |
| 13 | 2-OMe | H | 1m | 60 | 83 | 198–201/— [new] |
| 14 | 3-Br | H | 1n | 40 | 89 | 161–163/— [32] |
| 15 | 4-Cl | 4-OMe | 1o | 50 | 88 | 193–194/— [33b] |
| 16 | H | 4-Cl | 1p | 40 | 86 | 238–240/240–241 [34] |
| 17 | 4-Cl | 4-Cl | 1q | 30 | 87 | 253–255/228–229 [34] |
Moreover, the reusability of magnetically core–shell catalyst was affirmed upon the condensation of benzaldehyde, acetophenone, malononitrile and ammonium acetate in the optimized reaction conditions for nine attempts. After accomplishment of each run, ethanol was added to the reaction mixture and heated to dissolve and extract the desired product and un-reacted starting materials. Subsequently, the catalyst was separated from the reaction mixture by applying a simple external magnet, repetitively washed with ethanol, dried and reutilized for another assay.
The depicted resulting data in Fig. 1, have elucidated that the catalytic performance of the core–shell catalyst was preserved after nine runs without any meaningful loss of its initial activity in the yield and the reaction time. It is deserving of pointing out that the run nine of the catalyst reusability test was carried out under the nitrogen atmosphere to demonstrate the final stage of the plausible pathway mechanism (Fig. 2).
 | ||
| Fig. 1 The reusability survey of the catalyst upon the reaction of benzaldehyde, acetophenone, malononitrile and ammonium acetate. | ||
 | ||
| Fig. 2 The structures of the oxidant agent {Fe3O4@SiO2@(CH2)3Im}C(CN)3 (A) nano complex [Fe3O4–SiO2](CH2)3[Im-H]+[C(CN)3]− anion (B) with sp2 structure. | ||
Herein, let us follow the course of reaction and specially the final step in the following suggested mechanism (Fig. 2). Previously reported studies have been proposed aerobic auto oxidation of intermediate (7) to its corresponding 2-amino-3-cyanopyridines (1). In contrast to the previously reported mechanistic description for the final step of the above described organic synthesis,33 we believed that, this step might be progress through uncommon hydride transfer as well as Cannizzaro reaction (Scheme 3)37 and H2 releasing from tricyclic orthoamide which presented in Scheme 4.35 Very recently, we proposed an anomeric based oxidation for the final step in mechanistic pathways of 1,4-dihydropyrano-[2,3-c]-pyrazole36 and 2,4,6-triarylpyridine37 derivatives synthesis (Schemes 5 and 6). For improving of this idea, reaction was occurred under nitrogen atmosphere and in the absence of any molecular oxygen. It was detected that, the reaction progressed under atmosphere of nitrogen as well as normal reaction condition using oxygen. By considering the above-mentioned evidence, conversion of intermediate (7) to its corresponding 2-amino-3-cyanopyridines (1) might be happened through uncommon hydride transfer and releasing of molecular hydrogen (H2). The C–H bond is so weakend via electron donation from the nitrogen lone pairs into the anti-bonding of C–H (σ*C–H orbital) which it can be broken by reaction with a proton to give molecular hydrogen. To the best of our knowledge, this phenomena has been named as anomeric effect. Very recently, we have introduced a new term entitled “Anomeric Based Oxidation (ABO)” for the above mentioned facts so that oxidation of various substrates were occurred via releasing of molecular hydrogen.35,37 Fortunately, experimental evidences and theoretical studies confirmed that the final step of the synthetic pathway is proceeded via an anomeric based oxidation mechanism.
 | ||
| Scheme 3 The proposed mechanism for the in situ oxidation-reduction in Cannizzaro reaction through unusual hydride transfer via anomericeffect.37 | ||
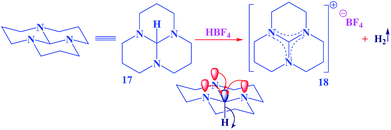 | ||
| Scheme 4 A striking example which had been observed for an unusual hydride transfer from tricyclic orthoamide (16) through anomeric effect.35 | ||
 | ||
| Scheme 5 An anomeric based oxidation for the final step in mechanistic pathways of 1,4-dihydropyrano-[2,3-c]-pyrazole derivatives synthesis.36 | ||
 | ||
| Scheme 6 An anomeric based oxidation for the final step in mechanistic pathways in the synthesis of 2,4,6-triarylpyridines using [SiO2–VO(OH)2 as SVA–H] as a catalyst.37 | ||
In a proposed mechanism, as portrayed in Scheme 7 the cationic site of the {Fe3O4@SiO2@(CH2)3Im}C(CN)3 plays a crucial role in activating of aromatic aldehydes and acetophenones as electrophile species and also at the same time the anionic site of the catalyst could interact with acidic hydrogen in malononitrile to trigger nucleophilic addition. Afterward, the reaction moves forward via the interaction between enamine 2 (generated from the reaction of ketone and ammonium acetate) and alkylidene malononitrile 3 (generated from the reaction of aldehyde and malononitrile) to yield the corresponding intermediate 4 followed by tautomerization and cyclization to give intermediate 6 which could tautomerize to intermediate 7 that has a suitable structure for anomeric based oxidation. As above mentioned, anomeric effect in intermediate 7(via –NH2 and –NH groups through C–C double bonds) leads to hydrid transfer, aromatization of the intermediate 7 and afford the related desired products 1a–q.
 | ||
| Scheme 7 Plausible mechanism for the synthesis of 2-amino-3-cyanopyridine derivatives in the presence of magnetically core–shell catalyst. | ||
In continuation of our previous investigations,36–38 herein, the modeling of the reactions of {Fe3O4@SiO2@(CH2)3Im}C(CN)3 as an oxidizing promoter catalyst with the R- and S-isomers of intermediate 7 performed on the basis of the anomeric effect of intermediate 7. For the calculations of this modeling have applied Spartan '10 package.39 The calculations on the structures of the R- and S-isomers of intermediate 7, the different positions of the transition states (TS) of the reactions, intermediate and the product have undertaken by DFT-B3LYP/6-31G* method.38 In Table 4 have shown the selected structural data (bond length (Å), bond angle (°) and torsional angle (°)) of the precursors (intermediate 7; R- and S-isomers), the transition states (with different orientations), intermediate and the product. Table 5 shows the calculated bond orders of the resonance process in intermediate 7 for the anomeric effect on C5–H6 bond in the two pathways. These two pathways are: N2C3C4C5H6 and N10C9C8C5H6. The N2C3C4C5H6 pathway of electron transfer has made a situation for electron ring currents and bring inside sextet for the heterocyclic ring. The N2C3C4C5H6 pathway of electron transfer (red pathway in figure of Table 5) includes: nN2 → π*C3–C4 and πC3–C4 → σ*C5–H6. The other electron transfer pathway (N2C9C8C5H6) includes: nN2 → π*C9–C8 and πC9–C8 → σ*C5–H6. The N10C9C8C5H6 pathway of electron transfer (blue pathway in figure of Table 5) includes: nN10 → π*C9–C8 and πC9–C8 → σ*C5–H6.
| Selected structural data | VSA | [TS] | Intermediate | Product | ||||
|---|---|---|---|---|---|---|---|---|
| R | S | R-out | R-in | S-out | S-in | |||
| Bond length (Å) | ||||||||
| H1–N2 | 1.013 | 1.012 | 1.010 | 1.010 | 1.010 | 1.010 | 1.014 | — |
| N2–C3 | 1.413 | 1.412 | 1.408 | 1.408 | 1.379 | 1.379 | 1.379 | 1.343 |
| C3–C4 | 1.345 | 1.345 | 1.347 | 1.347 | 1.369 | 1.369 | 1.378 | 1.403 |
| C5–C7 | 1.533 | 1.534 | 1.526 | 1.526 | 1.526 | 1.526 | 1.472 | 1.486 |
| C5–H6 | 1.102 | 1.100 | 1.671 | 1.611 | 1.671 | 1.611 | — | — |
| C5–C8 | 1.529 | 1.528 | 1.522 | 1.522 | 1.511 | 1.511 | 1.412 | 1.411 |
| C8–C9 | 1.368 | 1.368 | 1.369 | 1.369 | 1.347 | 1.347 | 1.419 | 1.428 |
| C9–N10 | 1.386 | 1.386 | 1.383 | 1.383 | 1.413 | 1.413 | 1.341 | 1.362 |
| C9–N2 | 1.511 | 1.513 | 1.379 | 1.379 | 1.408 | 1.408 | 1.360 | 1.337 |
| N1–C5′ | — | — | 1.381 | 1.381 | 1.381 | 1.381 | — | — |
| C5′–C4′ | — | — | 1.363 | 1.363 | 1.363 | 1.363 | — | — |
| C4′–N3 | — | — | 1.385 | 1.385 | 1.385 | 1.385 | — | — |
| N3–C2 | — | — | 1.334 | 1.334 | 1.334 | 1.334 | — | — |
| C2–N1 | — | — | 1.340 | 1.340 | 1.340 | 1.340 | — | — |
| H9–N1 | — | — | 1.652 | 1.652 | 1.652 | 1.652 | — | — |
| H9–H6 | — | — | 0.850 | 0.880 | 0.850 | 0.880 | — | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Bond angle (°) | ||||||||
| H1N2C3 | 115.42 | 116.25 | 117.66 | 117.66 | 116.44 | 116.44 | 116.84 | — |
| H1N2C4 | 114.50 | 115.52 | 116.44 | 116.44 | 117.66 | 117.66 | 118.89 | — |
| N2C2C4 | 120.71 | 120.52 | 119.83 | 119.83 | 121.25 | 121.25 | 117.79 | 122.10 |
| C3C4C5 | 124.63 | 124.37 | 123.54 | 123.54 | 121.12 | 121.12 | 121.91 | 120.25 |
| C4C5C6 | 108.63 | 108.25 | 109.43 | 109.43 | 106.43 | 106.43 | — | — |
| C7C5C6 | 106.05 | 106.15 | 103.45 | 103.45 | 103.45 | 103.45 | — | — |
| C5C8C9 | 122.67 | 122.53 | 121.12 | 121.12 | 123.54 | 123.54 | 120.12 | 118.40 |
| C8C9N10 | 124.62 | 124.74 | 124.56 | 124.56 | 124.84 | 124.84 | 122.89 | 120.93 |
| H9N1C2 | — | — | 125.83 | 125.83 | 124.72 | 124.72 | — | — |
| H9N1C5′ | — | — | 124.72 | 124.72 | 125.83 | 125.83 | — | — |
| N1C2N3 | — | — | 108.17 | 108.17 | 108.17 | 108.17 | — | — |
| N1C5′C4′ | — | — | 106.26 | 106.26 | 106.26 | 106.26 | — | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Dihedral angle (°) | ||||||||
| H1N2C3C4 | 149.31 | 149.66 | 148.89 | 148.89 | −152.75 | −152.75 | 175.35 | — |
| H1N2C9N10 | 27.43 | −33.61 | 26.21 | 26.21 | −31.29 | −31.29 | 4.54 | — |
| H1N2C9C8 | 44.73 | 43.62 | 40.94 | 40.94 | 148.89 | 148.89 | −175.22 | — |
| N2C3C4C5 | 178.92 | 175.80 | 10.84 | 10.84 | 10.84 | 10.84 | −0.40 | 1.40 |
| C3C4C5C7 | 122.69 | −117.69 | 143.94 | 143.94 | −146.60 | −146.60 | −179.49 | 179.57 |
| C3C4C5H6 | −120.89 | 126.13 | −101.44 | −101.44 | 100.12 | 100.12 | — | — |
| C3C4C5C8 | −1.90 | 8.30 | 10.33 | 10.33 | −18.50 | −18.50 | 1.53 | −0.97 |
| C4C5C8C9 | 0.89 | −9.32 | 164.22 | 164.22 | 15.33 | 15.33 | −1.44 | −0.29 |
| H1N2C9C8 | −44.23 | 42.16 | 40.94 | 40.94 | 148.89 | 148.89 | −175.22 | — |
| H9N1C2N3 | — | — | 179.38 | 179.38 | 179.38 | 17.38 | — | — |
| H9N1C5′C4′ | — | — | 0.61 | 0.61 | −179.56 | −179.56 | — | — |
| N1C2N3C4′ | — | — | 0.22 | 0.22 | 0.22 | 0.22 | — | — |
| Structures and pathways | Bond orders | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C9–N10 | C8–C9 | C5–C8 | C5–C7 | C5–H6 | C4–C5 | C3–C4 | N2–C3 | N2–C9 | H1–N2 | |
| R-isomer | 1.04 | 1.57 | 0.97 | 0.95 | 0.91 | 0.97 | 1.77 | 0.91 | 0.99 | 0.87 |
| S-isomer | 1.04 | 1.56 | 0.98 | 0.95 | 0.93 | 0.97 | 1.77 | 0.97 | 0.99 | 0.88 |
| R-NH | 1.03 | 1.55 | 0.97 | 0.94 | 0.93 | 0.97 | 1.76 | 0.97 | 0.99 | 0.89 |
| S-NH | 1.03 | 1.55 | 0.98 | 0.94 | 1.10 | 0.97 | 1.77 | 0.97 | 0.99 | 0.89 |
| R-NH2 | 1.03 | 1.55 | 0.97 | 0.94 | 1.10 | 0.97 | 1.77 | 0.97 | 0.99 | 0.89 |
| S-NH2 | 1.04 | 1.56 | 0.98 | 0.95 | 1.10 | 0.97 | 1.77 | 0.97 | 0.99 | 0.88 |
In Fig. 2 were demonstrated the structures of the oxidizing promoter catalyst [Fe3O4–SiO2](CH2)3[Im-H]+ and [C(CN)3]−. The model of nano-structure complex {Fe3O4@SiO2@(CH2)3Im}C(CN)3 was shown in Fig. 2A. The DFT calculations demonstrated that the structure of the anion is planar and the central atom of [C(CN)3]− anion has sp2 structure. The Mulliken charges on N-atom in [C(CN)3]− is more than five times greater than central C-atom. So, it is possible that these N-atoms act as base agent to attract H+ from the intermediate at the next step of the reaction.
Fig. 3 shows the optimized structures of the R- and S-isomers of intermediate 7. The R-isomer and S-isomer have trans- and cis-structures, respectively. The DFT-B3LYP/6-31G* calculations has demonstrated that the R-isomer of intermediate 7 is 0.29 kcal mol−1 is less stable than its S-isomer. The comparison of the free activation energies (ΔG#) demonstrates that R-isomer is more reactive than S-isomer.
The calculated structures of the transition states ([TS]R and [TS]S) for the first step of the anomeric based oxidation reaction on the basis of anomeric effects in R- and S-isomers of intermediate 7 with {Fe3O4@SiO2@(CH2)3Im}C(CN)3 were presented in Fig. 3A and B. The bond lengths of C5–H6, H6–C9 and H6⋯C9 in the more stable transition state were obtained as: 1.671, 1.652 and 0.850 Å, respectively. See Table 4. The DFT calculations shows that the structure (A-out orientation) is more stable than (B-in orientation) for [TS]R and [TS]S.
In Fig. 5 has presented the structure of the H+ attraction step from intermediate 7 by [C(CN)3]− anion of {Fe3O4@SiO2@(CH2)3Im}C(CN)3 agent. Because of the higher Mulliken charge on N-atoms in [C(CN)3]− anion the N-atoms attracts the proton in the intermediate. See Fig. 1B and 4.
The reaction coordinate process for changing the C5⋯H6 (intermediate 7) and N1⋯H9 (oxidizing promoter catalyst) bonds lengths was demonstrated in Fig. 6. The three dimensional diagram on the DFT calculations was designed to obtain the transition state point [TSR] of the out orientation. The calculated ZPE free activation energy (ΔG#) for [TSR] of the out orientation is 33.85 kcal mol−1 (with bond lengths of C5–H6, H6–C9 and H6⋯C9 as: 1.671, 1.652 and 0.850 Å, respectively).
The reaction diagram and free energies (in kcal mol−1) of intermediate 7 oxidation reaction on the basis of anomeric effect by {Fe3O4@SiO2@(CH2)3Im}C(CN)3 agent were shown in Fig. 7 (for out-(A) and in-(B) orientations see Fig. 3). The oxidation reaction for out orientation (with ΔG# = 33.85 kcal mol−1) has the highest reaction rate among the different calculated situations. See the table of Fig. 7. R-isomer is 0.29 kcal mol−1 less stable than R-isomer of intermediate 7 but the oxidation reaction free activation energy (ΔG#) for [TSR] that has out orientation is lower than S-isomer. As mentioned before, the calculated bond order of C5–H6 for R-isomer is 0.02 less that S-isomer and it could be the reason of the reactivity willingness of R-isomer than S-isomer on the basis of the anomeric effect in intermediate 7 structure. The calculated free energies of the reactant and the product (ΔG1) of the oxidation reactions for R- and S-isomers of intermediate 7 were obtained −19.27 and −18.98 kcal mol−1, respectively. Because of the same intermediate in this reaction the ΔG were same for these different oxidation reactions. The calculated free energies of the reactant and the intermediate (ΔG2) form of the oxidation reactions of the R- and S-isomers were obtained −12.52 and −12.33 kcal mol−1, respectively. See free energies table of Fig. 7. In notice to the results of the DFT-B3LYP/6-31G*, S-isomer is more stable than R-isomer of intermediate 7. But, R-isomer because of the lower bond order of C5–H6 due to the anomeric effect has more rate of the oxidation reaction with lower free activation energy (ΔG#).
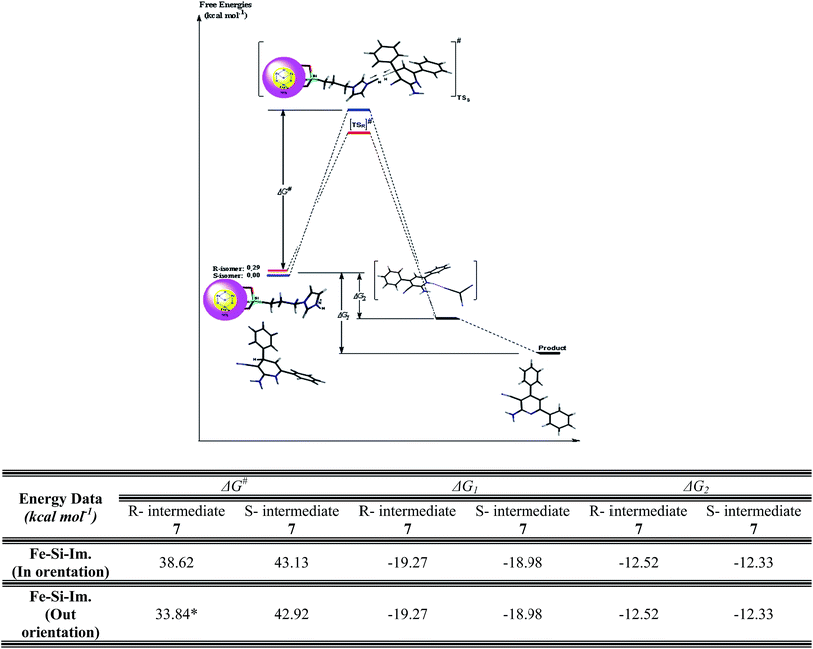 | ||
| Fig. 7 The reaction diagram and free energies (in kcal mol−1) of intermediate 7 oxidation reaction on the basis of anomeric effect by {Fe3O4@SiO2@(CH2)3Im}C(CN)3 agent. For out-(A) and in-(B) orientations see Fig. 4. *The rate of the reaction for out orientation is the highest among the situations. | ||
Conclusion
In conclusion, in this study, we have presented a novel and effective one-pot protocol for the synthesis of 2-amino-4,6-diphenylnicotinonitrile derivatives in the presence of silica-coated magnetic nanoparticles with a tag of ionic liquid namely {Fe3O4@SiO2@(CH2)3Im}C(CN)3 as a heterogeneous catalyst under solvent free and benign conditions. The reaction was performed using a good range of aromatic aldehydes, acetophenone derivatives, malononitrile and ammonium acetate to yield the related desired products in relatively short reaction times with good to high yields. It is suggested that the final step of the plausible mechanism in the synthetic pathway to target molecules goes on via an anomeric based oxidation mechanism which was confirmed by described theoretical studies.Experimental
General
All reagents and materials were procured from Fluka and Merck chemical companies. The known products were identified by comparison of their physical properties and spectral data with their reported authentic samples in the literature. The reaction progress and the purity of the compounds were verified using TLC performed with silica gel SIL G/UV 254 plates. NMR spectra were recorded on a Bruker spectrometer, 1H NMR (400.13) and 13C NMR (100.62). The data for 1H NMR are reported as follows: chemical shift (ppm), integration, multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; and br, broad), coupling constant (Hz). Melting points were recorded on Buchi B-545 apparatus in open capillary tubes.General procedure for the synthesis of 2-amino-4,6-diphenylnicotinonitrile derivatives (Scheme 1)
To a round bottom flask containing a mixture of aromatic aldehydes (1 mmol), acetophenone derivatives (1 mmol), malononitrile (1 mmol, 0.066 g), and ammonium acetate (3 mmol, 0.23 g), amount of 7 mg of silica-coated magnetic nanoparticles with a tag of ionic liquid ({Fe3O4@SiO2@(CH2)3Im}C(CN)3) was added as a heterogeneous core–shell catalyst. Then, the mixture was vigorously stirred for proper time as embedded in Table 3 under solvent free condition at 100 °C. The progress of the reaction was checked using TLC with a mixture of n-hexane and ethylacetate as the eluent system. After completion of the reaction, the mixture was cooled to room temperature. Subsequently, in order to separation of the catalyst, hot ethanol was added to the reaction mixture and the magnetic core–shell catalyst was separated using a simple external magnet. At the end, the products were recrystallized from ethanol with good to high yields as depicted in Table 2.Spectral data
FT-IR (KBr): ν (cm−1) = 3466, 3305, 3180, 2206, 1638, 1573, 1423, 1370, 1259, 755, 698.
δ H (400 MHz, DMSO) 8.14 (s, 2H, aromatic), 7.52 (m, 8H, aromatic), 7.29 (s, 1H, aromatic), 7.06 (s, 2H, NH2).
δ C (101 MHz, DMSO) 160.9, 128.6, 154.9, 137.5, 137.0, 130.1, 129.6, 128.7, 128.6, 128.3, 127.2, 117.0, 109.2, 86.6.
FT-IR (KBr): ν (cm−1) = 3498, 3475, 3389, 3311, 3190, 2207, 1645, 1632, 1578, 1548, 1492, 1259, 816, 697.
δ H (400 MHz, DMSO) 8.14–8.12 (m, 2H, aromatic), 7.72 (d, J = 8.6 Hz, 2H, aromatic), 7.63 (d, J = 8.6 Hz, 2H, aromatic), 7.51–7.48 (m, 3H, aromatic), 7.29 (s, 1H, aromatic), 7.08 (s, 2H, NH2).
δ C (101 MHz, DMSO) 160.8, 158.7, 153.6, 137.4, 135.8, 134.5, 130.3, 130.2, 128.7, 128.6, 127.2, 116.9, 109.1, 86.4.
FT-IR (KBr): ν (cm−1) = 3466, 3308, 3182, 2206, 1639, 1575, 1515, 1255, 1181, 824, 769.
δ H (400 MHz, DMSO) 8.13–8.11 (m, 2H, aromatic), 7.66 (d, J = 8.6 Hz, 2H), 7.49–7.48 (m, 3H, aromatic), 7.25 (s, 1H, aromatic), 7.11 (d, J = 8.7 Hz, 2H, aromatic), 6.97 (s, 2H, NH2), 3.84 (s, 3H, OMe).
δ C (101 MHz, DMSO) 160.9, 160.4, 158.4, 154.4, 137.6, 130.0, 129.8, 129.0, 128.6, 127.2, 117.3, 114.1, 109.0, 86.3, 55.3.
FT-IR (KBr): ν (cm−1) = 3489, 3342, 3214, 2229, 1624, 1572, 1554, 1253, 764, 688.
δ H (400 MHz, DMSO) 8.11 (m, 2H, aromatic), 7.66 (d, J = 7.2 Hz, 1H), 7.55–7.48 (m, 6H, aromatic), 7.22 (s, 1H, aromatic), 7.12 (s, 2H, NH2).
δ C (101 MHz, DMSO) 160.1, 158.5, 153.0, 137.2, 136.2, 131.2, 130.9, 130.7, 130.2, 129.7, 128.7, 127.5, 127.2, 116.1, 109.7, 88.4.
FT-IR (KBr): ν (cm−1) = 3490, 3373, 3242, 2210, 1652, 1638, 1573, 1349, 852, 679.
δ H (400 MHz, DMSO) 8.38 (s, 2H, aromatic), 8.15 (s, 2H, aromatic), 7.96 (s, 2H, aromatic), 7.65–6.88 (m, 6H, aromatic and NH2).
δ C (101 MHz, DMSO) 160.7, 158.9, 152.7, 143.3, 137.3, 130.2, 130.01, 129.6, 128.7, 128.6, 127.3, 123.7, 118.4, 109.1.
FT-IR (KBr): ν (cm−1) = 3475, 3311, 3182, 2207, 1646, 1575, 1512, 1235, 831, 697.
δ H (400 MHz, DMSO) 8.14–8.12 (m, 2H, aromatic), 7.77–7.74 (m, 2H, aromatic), 7.51–7.48 (m, 3H, aromatic), 7.41 (t, J = 8.9 Hz, 2H), 7.29 (s, 1H, aromatic), 7.05 (s, 2H, NH2).
δ C (101 MHz, DMSO) 160.8, 158.6, 153.8, 137.5, 133.3, 130.8, 130.7, 130.1, 128.6, 127.2, 117.0, 115.9, 115.6, 109.2.
FT-IR (KBr): ν (cm−1) = 3486, 3351, 3223, 2216, 1631, 1573, 1545, 1259, 824, 687.
δ H (400 MHz, DMSO) 8.13 (s, 2H, aromatic), 7.76 (s, 2H, aromatic), 7.65–7.49 (m, 5H, aromatic), 7.29 (s, 1H, aromatic), 7.08 (s, 2H, NH2).
δ C (101 MHz, DMSO) 160.8, 159.2, 153.7, 137.4, 136.1, 135.4, 131.7, 130.5, 130.2, 128.6, 127.2, 116.8, 116.1, 114.9, 109.0.
FT-IR (KBr): ν (cm−1) = 3344, 3363, 3226, 2225, 2213, 1663, 1552, 1529, 813, 704.
δ H (400 MHz, DMSO) 9.24 (d, J = 59.6 Hz, 2H), 7.74–7.67 (m, 4H, aromatic), 7.63 (s, 1H, aromatic), 7.48–7.45 (m, 2H, aromatic), 7.41 (d, J = 8.0 Hz, 2H), 6.83 (d, J = 15.7 Hz, 1H), 2.50 (s, 3H, Me).
δ C (101 MHz, DMSO) 164.4, 161.2, 144.8, 140.9, 132.9, 131.7, 130.2, 129.8, 128.5, 128.4, 128.3, 124.2, 115.5, 114.9, 113.7, 21.0.
FT-IR (KBr): ν (cm−1) = 3480, 3364, 3224, 2218, 1622, 1579, 1530, 1344, 723, 691.
δ H (400 MHz, DMSO) 8.68 (s, 1H, aromatic), 8.55 (s, 1H, aromatic), 8.32 (bs, 3H, aromatic), 8.03 (s, 1H, aromatic), 7.66–7.58 (m, 4H, aromatic), 7.33 (s, 2H, NH2).
δ C (101 MHz, DMSO) 160.8, 158.9, 152.5, 147.8, 135.1, 130.4, 130.3, 128.6, 127.3, 124.3, 123.3, 116.7, 109.3.
FT-IR (KBr): ν (cm−1) = 3469, 3359, 3225, 2203, 1619, 1584, 1224, 762, 694.
δ H (400 MHz, DMSO) 9.94 (s, 1H, OH), 8.0 (s, 2H, aromatic), 7.65 (s, 2H, aromatic), 7.54 (s, 4H, aromatic), 7.16 (s, 1H, aromatic), 6.91–6.84 (m, 4H, aromatic and NH2).
δ C (101 MHz, DMSO) 160.8, 159.5, 158.6, 154.5, 137.2, 129.4, 128.9, 128.7, 128.3, 117.3, 115.4, 108.1, 85.2.
FT-IR (KBr): ν (cm−1) = 3466, 3364, 3238, 2215, 1643, 1634, 1578, 1286, 842, 696.
δ H (400 MHz, DMSO) 8.01–7.86 (m, 4H, aromatic), 7.64–7.52 (m, 5H, aromatic), 7.15–6.85 (m, 3H, aromatic and NH2).
δ C (101 MHz, DMSO) 154.0, 150.1, 148.0, 141.9, 137.2, 132.6, 129.7, 129.6, 128.7, 128.6, 118.4, 115.8, 115.7, 112.1, 93.8.
FT-IR (KBr): ν (cm−1) = 3461, 3352, 3228, 2208, 1622, 1578, 1363, 1232, 805.
δ H (400 MHz, DMSO) 9.96 (s, 1H, OH), 8.00 (s, 2H, aromatic), 7.65 (d, J = 22.4 Hz, 1H), 7.17 (s, 1H, aromatic), 6.96 (s, 2H, aromatic), 6.85 (s, 2H, NH2).
δ C (101 MHz, DMSO) 160.7, 159.6, 158.7, 153.2, 136.0, 134.3, 130.2, 129.0, 128.7, 128.2, 126.5, 117.1, 115.4, 108.0, 85.0.
FT-IR (KBr): ν (cm−1) = 3483, 3343, 2227, 1623, 1552, 1444, 1365, 1251, 761, 686.
δ H (400 MHz, DMSO) 8.09 (s, 2H, aromatic), 7.48 (s, 4H, aromatic), 7.35 (s, 1H, aromatic), 7.18 (s, 2H, aromatic),7.10 (s, 1H, aromatic), 6.95 (s, 2H, NH2), 3.81 (s, 3H, OMe).
δ C (101 MHz, DMSO) 160.1, 158.3156.0, 152.9, 137.6, 130.9, 130.0, 129.9, 128.6, 127.1, 126.1, 120.6, 116.7, 111.7, 110.2, 89.1, 55.4.
m/z calcd for C19H15N3O = 301.35, found = 301.3.
FT-IR (KBr): ν (cm−1) = 3480, 3336, 3211, 2224, 1622, 1570, 1548, 1255, 768, 686.
δ H (400 MHz, DMSO) 8.15 (d, J = 3.8 Hz, 2H), 7.89 (s, 1H, aromatic), 7.72 (dd, J = 21.0, 7.7 Hz, 2H), 7.54–7.49 (m, 4H, aromatic), 7.32 (s, 1H, aromatic), 7.10 (s, 2H, NH2).
δ C (101 MHz, DMSO) 160.7, 158.8, 153.2, 139.2, 137.4, 132.3, 130.9, 130.8, 130.2, 128.6, 127.5, 127.3, 121.9, 116.8, 109.2, 86.5.
FT-IR (KBr): ν (cm−1) = 3459, 3364, 2206, 1634, 1546, 1440, 1238, 826, 635.
δ H (400 MHz, DMSO) 8.11 (s, 2H, aromatic), 7.64–7.70 (m, 4H, aromatic), 7.24 (s, 1H, aromatic), 7.03 (s, 3H, aromatic and NH2), 3.83 (s, 3H, OMe).
δ C (101 MHz, DMSO) 161.0, 160.7, 158.4, 153.3, 135.9, 134.4, 130.2, 129.7, 128.8, 128.7, 117.1, 114.0, 108.3, 85.4, 55.3.
FT-IR (KBr): ν (cm−1) = 3500, 3395, 2209, 1610, 1571, 1549, 1088, 831, 695.
δ H (400 MHz, DMSO) 8.17 (s, 2H, aromatic), 7.68–7.55 (m, 7H, aromatic), 7.32 (s, 1H, aromatic), 7.09 (s, 2H, NH2).
δ C (101 MHz, DMSO) 160.8, 157.2, 155.1, 148.8, 144.5, 136.8, 136.3, 134.9, 129.6, 129.0, 128.7, 128.7, 128.4, 116.9, 109.2, 86.9.
FT-IR (KBr): ν (cm−1) = 3501, 3396, 2206, 1609, 1581, 1546, 1094, 823.
δ H (400 MHz, DMSO) 8.17 (d, J = 7.9 Hz, 2H), 7.68 (dd, J = 33.0, 7.8 Hz, 4H), 7.57 (d, J = 8.0 Hz, 2H)., 7.32 (s, 1H, aromatic), 7.12 (s, 2H, NH2).
δ C (101 MHz, DMSO) 160.7, 157.3, 153.8, 136.2, 135.6, 135.0, 134.5, 130.3, 129.0, 128.7, 128.7, 116.7, 109.1, 86.8.
Acknowledgements
We thank Bu-Ali Sina University and Iran National Science Foundation (INSF) for financial support (The Grant of Allameh Tabataba'i's Award, Grant Number: BN093) to our research group.References
- N. G. Kozlov and A. P. Kadutskii, Tetrahedron Lett., 2008, 49, 560 Search PubMed.
- D. Tejedor and F. Garcia-Tellado, Chem. Soc. Rev., 2007, 36, 484 RSC.
- A. Zare, F. Abi, A. R. Moosavi-Zare, M. H. Beyzavi and M. A. Zolfigol, J. Mol. Liq., 2013, 178, 113 CrossRef CAS.
- G. D. Henry, Tetrahedron, 2004, 60, 6043 CrossRef CAS.
- J. P. Michael, Natural Products Reports, 2005, 22, 627 RSC.
- M. Movassaghi, M. D. Hill and O. K. Ahmad, J. Am. Chem. Soc., 2007, 129, 10096 CrossRef CAS PubMed.
- T. Murata, M. Shimada, S. Sakakibara, T. Yoshino, H. Kadono, T. Masuda, M. Shimazaki, T. Shintani, K. Fuchikami, K. Sakai, H. Inbe, K. Takeshita, T. Niki, M. Umeda, K. B. Bacon, K. B. Ziegelbauer and T. B. Lowinger, Bioorg. Med. Chem. Lett., 2003, 13, 913 CrossRef CAS PubMed.
- M. Mantri, O. De Graaf, J. Van Veldhoven, A. Göblyös, J. K. Von Frijtag Drabbe Künzel, T. Mulder-Krieger, R. Link, H. De Vries, M. W. Beukers, J. Brussee and A. P. Ijzerman, J. Med. Chem., 2008, 51, 4449 CrossRef CAS PubMed.
- J. Deng, T. Sanchez, L. Q. Al-Mawsawi, R. Dayam, R. A. Yunes, A. Garofalo, M. B. Bolger and N. Neamati, Bioorg. Med. Chem., 2007, 15, 4985 CrossRef CAS PubMed.
- F. Shi, V. Tu, F. Fang and T. Li, ARKIVOC, 2005, 137 CAS.
- S. J. Tu, H. Jiang, Q. Y. Zhuang, C. B. Miao, D. Q. Shi, X. S. Wang and Y. C. Gao, Chin J. Org. Chem., 2003, 23, 488 CAS.
- W. J. Zhou, S. J. Ji and Z. L. Shen, J. Organomet. Chem., 2006, 691, 1356 CrossRef CAS.
- R. Gupta, A. Jain, M. Jain and R. Joshi, Bull. Korean Chem. Soc., 2010, 31, 3180 CrossRef CAS.
- T. S. Jin, A. Q. Wang, F. Shi, L. S. Han, L. B. Liu and T. S. Li, ARKIVOC, 2006, xiv, 78 Search PubMed.
- A. M. Shestopalov and O. A. Naumov, Russ. Chem. Bull., 2003, 52, 1380 CrossRef CAS.
- A. N. Vasiliev, Y. S. Kayukov, O. E. Nasakin, A. N. Lyshchikov, V. N. Nesterov, O. V. Kayukova and O. V. Poulkherovskaya, Chem. Heterocycl. Compd., 2001, 37, 309 CrossRef CAS.
- M. M. Heravi, S. Y. S. Beheshtiha, M. Dehghani and N. Hosseintash, J. Iran. Chem. Soc., 2015, 12, 2075–2081 CrossRef CAS.
- S. S. Mansoor, K. Aswin, K. Logaiya, P. N. Sudhan and S. Malik, Res. Chem. Intermed., 2014, 40, 871 CrossRef CAS.
- J. Tang, L. Wang, Y. Yao, L. Zhang and W. Wang, Tetrahedron Lett., 2011, 52, 509 CrossRef CAS.
- S. Kambe and K. Saito, Synthesis, 1980, 366 CrossRef CAS.
- A. S. Girgis, A. Kalmouch and H. M. Hosni, Amino Acids, 2004, 26, 139 CrossRef CAS PubMed.
- F. Shi, S. J. Tu, F. Fang and T. J. Li, ARKIVOC, 2005, 137 CAS.
- P. Gothawal, G. Malhotra and Y. K. Srivastava, Eur. J. Chem., 2011, 8, 119 Search PubMed.
- See our review: (a) P. Salehi, M. A. Zolfigol, F. Shirini and M. Baghbanzadeh, Curr. Org. Chem., 2006, 10, 2171 CrossRef CAS; (b) M. Daraei, M. A. Zolfigol, F. Derakhshan-Panah, M. Shiri, H. G. Kruger and M. Mokhlesi, J. Iran. Chem. Soc., 2015, 12, 855 CrossRef CAS; (c) D. Azarifar, S. M. Khatami, M. A. Zolfigol and R. Nejat-Yami, J. Iran. Chem. Soc., 2014, 11, 1223 CrossRef CAS; (d) A. Khazaei, M. A. Zolfigol, M. Mokhlesi and R. Rostamian, J. Iran. Chem. Soc., 2013, 10, 1297 CrossRef CAS.
- See our review: F. Shirini, M. A. Zolfigol, P. Salehi and M. Abedini, Curr. Org. Chem., 2008, 12, 183 CrossRef CAS.
- (a) A. R. Moosavi-Zare, M. A. Zolfigol, M. Zarei, A. Zare and J. Afsar, Appl. Catal., A, 2015, 505, 224 CrossRef CAS; (b) A. Zare, M. Merajoddin, A. R. Moosavi-Zare, M. Zarei, M. H. Beyzavi and M. A. Zolfigol, Res. Chem. Intermed., 2016, 42, 2365 Search PubMed.
- (a) A. R. Moosavi-Zare, M. A. Zolfigol, V. Khakyzadeh, C. Böttcher, M. H. Beyzavi, A. Zare, A. Hasaninejad and R. Luque, J. Mater. Chem. A., 2014, 2, 770 RSC; (b) M. A. Zolfigol, S. Baghery, A. R. Moosavi-Zare and S. M. Vahdat, RSC Adv., 2015, 5, 32933 RSC; (c) M. A. Zolfigol, S. Baghery, A. R. Moosavi-Zare, S. M. Vahdat, H. Alinezhad and M. Norouzi, RSC Adv., 2015, 5, 45027 RSC; (d) M. A. Zolfigol, S. Baghery, A. R. Moosavi-Zare and S. M. Vahdat, J. Mol. Catal. A: Chem., 2015, 409, 216 CrossRef CAS; (e) M. A. Zolfigol, R. Ayazi-Nasrabadi and S. Baghery, RSC Adv., 2015, 5, 71942 RSC; (f) M. A. Zolfigol, S. Baghery, A. R. Moosavi-Zare, S. M. Vahdat, H. Alinezhad and M. Norouzi, RSC Adv., 2014, 4, 57662 RSC.
- See our reviews: (a) E. Kolvari, A. Ghorbani-Choghamarani, P. Salehi, F. Shirini and M. A. Zolfigol, J. Iran. Chem. Soc., 2007, 4, 126 CrossRef CAS; (b) H. Veisi, R. Ghorbani-Waghei and M. A. Zolfigol, Org. Prep. Proced. Int., 2011, 43, 489 CrossRef CAS; (c) E. Kolvari, N. Koukabi, A. Khoramabadi-zad, A. Shiri and M. A. Zolfigol, Curr. Org. Synth., 2013, 10, 837 CrossRef CAS.
- M. A. Zolfigol and M. Yarie, RSC Adv., 2015, 5, 103617 RSC.
- Q. Wu, Y. Zhang and S. Cui, Org. Lett., 2014, 16, 1350 CrossRef CAS PubMed.
- C. Kurumurthy, R. Naresh Kumar, T. Yakaiah, P. Shanthan Rao and B. Narsaiah, Res. Chem. Intermed., 2015, 41, 3193 CrossRef CAS.
- R. Bodireddy Mohan, N. C. Gangi Reddy and S. D. Kumar, RSC Adv., 2014, 4, 17196 RSC.
- (a) J. Safari, S. H. Banitab and S. D. Khalili, Ultrason. Sonochem., 2012, 19, 1061 CrossRef CAS PubMed; (b) S. Khaksar and M. Yaghoobi, J. Fluorine Chem., 2012, 142, 41 CrossRef CAS.
- H. C. Shah, V. H. Shah and N. D. Desai, ARKIVOC, 2009, ii, 76 Search PubMed.
- (a) J. M. Erhardt and J. D. Wuest, J. Am. Chem. Soc., 1980, 102, 6363 CrossRef CAS; (b) T. J. Atkins, J. Am. Chem. Soc., 1980, 102, 6364 CrossRef CAS; (c) J. M. Erhardt, E. R. Grover and J. D. Wuest, J. Am. Chem. Soc., 1980, 102, 6365 CrossRef CAS.
- M. A. Zolfigol, F. Afsharnadery, S. Baghery, S. Salehzadeh and F. Maleki, RSC Adv., 2015, 5, 75555 RSC.
- M. A. Zolfigol, M. Safaiee, F. Afsharnadery, N. Bahrami-Nejad, S. Baghery, S. Salehzadeh and F. Maleki, RSC Adv., 2015, 5, 100546 RSC.
- S. A. Glover, A. A. Rosser, A. Taherpour and B. W. Greatrex, Aust. J. Chem., 2014, 67, 507 CrossRef CAS.
- All of the calculations were performed by: Spatran'10-Quantum Mechanics Program: (PC/x86) 1.1.0v4. 2011, Wavefunction Inc., USA.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12299j |
| This journal is © The Royal Society of Chemistry 2016 |






