One-pot synthesis of fused benzoxazino[1,2,3]triazolyl[4,5-c]quinolinone derivatives and their anticancer activity†
Sirassu Narsimhaa,
Kumara Swamy Battulaa,
Satheesh Kumar Nukalaa,
Ramesh Gondrub,
Yellu Narasimha Reddyc and
Vasudeva Reedy Nagavelli*a
aDepartment of Chemistry, Kakatiya University, Warangal, TS 506 009, India. E-mail: vasujac3@gmail.com; Tel: +91-9949023869
bDepartment of Chemistry, National Institute of Technology, Warangal – 506 004, Telangana State, India
cDepartment of Pharmacology and Toxicology, Kakatiya University, Warangal, TS 506 009, India
First published on 22nd July 2016
Abstract
A one pot strategy for the synthesis of fused benzoxazino[1,2,3]triazolyl[4,5-c]quinolinone derivatives 4a–4k via the reaction of 5-iodo-4-(prop-2-yn-1-yl)-2H-benzo[b][1,4]oxazin-3(4H)-one (3) with different aryl azides under copper and palladium catalysis in a ligand free condition is achieved. The reaction provided the desired fused triazoles in good to excellent yields. The synthesized derivatives were evaluated for their in vitro cytotoxic activity against MCF-7, A-549 and HeLa cancer cell lines using MTT assay. The cytotoxic activity results revealed that, compound 4d has shown a broad spectrum of activity against MCF-7 and A-549 with IC50 values of 11.18 ± 0.8 and 17.81 ± 0.6 μM, which are comparable to the standard drug, cisplatin. The remaining compounds have shown good to moderate activity against the tested cell lines. Based on the results obtained, a structure activity relationship (SAR) is discussed.
Introduction
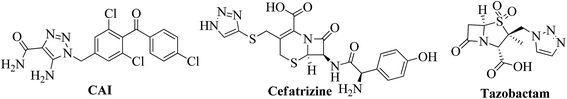
Substituted 1,2,3-triazoles are indispensable structural motifs of compounds that display a broad spectrum of biological activities, and are widely used in organic, medicinal, and material science.1 Among them, disubstituted (1,4 & 1,5) and 1,4,5-trisubstituted 1,2,3-triazoles were found to have a wide range of pharmaceutical applications.2 For example, the 1,2,3-triazole moiety is found in clinically used drugs including, β-lactam antibiotics, tazobactam,3 cefatrizine,4 and a calcium channel blocker carboxyamidotriazole (CAI)5 (Fig. 1). On the other hand, the chemistry of 1,4-benzoxazines and their fused heterocyclic scaffolds has received considerable attention owing to their synthetic and effective biological importance.6 For example, a large number of 1,4-benzoxazine derivatives (Fig. 2) have been incorporated into a wide variety of therapeutically interesting drug candidates possessing antibacterial,7 antifungal,8 anticancer,9 and anticonvulsant activities10 and into therapeutic agents in the treatment of cardiovascular diseases.11A common approach for the synthesis of 1,2,3-triazoles is the copper-catalyzed Huisgen cycloaddition of alkynes with azides (CuAAC). However, the regioselective synthesis of fused 1,4,5-trisubstituted 1,2,3-triazoles from terminal alkynes has been established to be a very challenging assignment. Recently the palladium-catalyzed direct arylation of triazoles via C–H functionalization by using aryl halides and direct oxidative arylations with unfunctionalized arene systems has led to success.12 One-pot synthesis of fused triazolo[4,5-d]quinoline derivatives via in situ generation of 1,4-disubstituted 1,2,3-triazoles from alkynes and aryl azides minimizes the hazards derived from their separation and handling. Jeh-Jeng Wang et al. reported fused triazolo[4,5-d]quinoline/chromene/thiochromene derivatives via palladium catalysis mediated by tetrabutylammonium iodide.13 Also in 2010, Mark Lautens and co-workers reported copper and palladium-catalyzed intramolecular C–H arylation of in situ generated 1,4-disubstituted triazoles, which set the stage for the development of a modular one-pot approach to fused triazolo[4,5-d]quinoline derivatives by using non-symmetrical internal 1-iodoalkynes.14 In 2012, Ackermann and their group synthesized annulated 1,2,3-triazoles through copper-catalyzed intramolecular C–H arylation.15
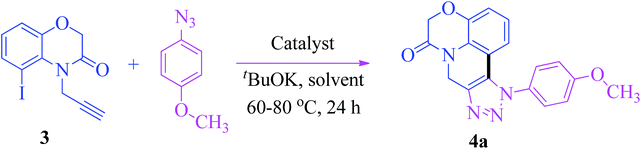
Encouraged by the aforementioned successful synthesis of fused 1,2,3-triazoles via intramolecular C–H arylation of in situ generated 1,4-disubstituted triazoles and keeping in view their versatile therapeutic properties, as well as in continuation of our research on 1,2,3-triazoles,16 herein, we report an efficient method for the synthesis of fused benzoxazino [1,2,3]triazolyl[4,5-c]quinolinone derivatives by using copper and palladium catalyzed intramolecular C–H arylation of in situ generated 1,4-disubstituted triazoles from 5-iodo-4-(prop-2-yn-1-yl)-2H-benzo[b][1,4]oxazin-3(4H)-one (3) and aryl azides in a one pot method. We further assessed these derivatives for their in vitro cytotoxic activity (Fig. 3).
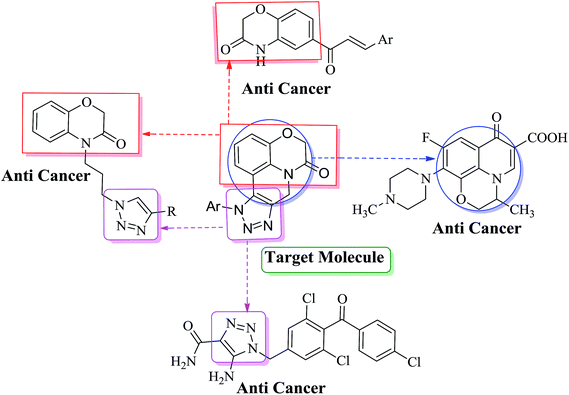 | ||
| Fig. 3 Representative examples of anticancer active heterocyclic molecules with benzoxazine, triazole and oxazino[4,5-c]quinolinone moieties. | ||
Results and discussion
The synthetic procedure adopted to obtain the target compounds is shown in Scheme 1. Our initial investigation began with the intramolecular cyclization of in situ generated 1,4-disubstituted triazoles from 5-iodo-4-(prop-2-yn-1-yl)-2H-benzo[b][1,4]oxazin-3(4H)-one (3) and 4-methoxyphenyl azide by following the previously reported literature conditions.15 However, the reaction did not proceed and the desired product 4a was not obtained (Table 1, entry 1). Since it was known that the use of palladium catalyst substantially improves the yields of many cross-coupling reactions,12 we decided to explore the application of such catalyst to our reaction. When the reaction was carried out with equimolar ratios of CuI (5 mol%) and Pd(PPh3)4 (5 mol%) in the presence of DMF, toluene and DMSO, the desired compound 4a was produced in below 30% yields (Table 1, entries 2–4). Surprisingly, the reaction of 3 with Pd (OAc)2 (5 mol%) and CuI (5 mol%) in the presence of tBuOK in DMF produced the desired compound 4a in a 51% yield (Table 1, entry 5). The results encouraged us to investigate further promising conditions to achieve better reaction yields. To this end we have carried out the reaction with 10 mol% CuI and 5 mol% Pd(OAc)2 in DMF, in the presence of tBuOK, and we have found that the yield of the desired product 4a was 56% and there was not much affect of the catalytic ratio of CuI on the yield of the product (Table 1, entry 6). From these results we have expected that increasing the ratio of Pd(OAc)2 may enhance the rate of the reaction. Based on the above results, we performed the reaction for further investigation of the catalytic effect of Pd(OAc)2 by increasing its catalytic load from 5 mol% to 10 mol%. The result showed that the increase in the catalyst loading enhances the product yield to 76% (Table 1, entry 7). It was also found that a further increase in the catalytic loading of Pd(OAc)2 to 15 mol% did not have much affect on the yield of the desired products (yield 79%) (Table 1, entry 8). After the complete optimization studies, it was clear that the use of catalytic loads of CuI (10 mol%) and Pd(OAc)2 (10 mol%) with 1 equivalent of tBuOK in DMF at 60–80 °C was the optimal reaction condition to achieve the final desired product in a good yield.| Entry | Catalyst | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reactions were performed with 3 (1.0 mmol), azide (1.0 mmol), and solvent (10 mL) at 80 °C for 30 h.b Isolated yield.c Ref. 15.d CuI/Pd (5 mol%) & tBuOK (1.0 mmol).e Cu/Pd (5 mol%).f Cu (10 mol%)/Pd (5 mol%).g Cu/Pd (10 mol%).h Cu (10 mol%)/Pd (15 mol%); N.R. = no reaction. | |||
| 1c | CuI | DMF | N.R. |
| 2d | CuI/Pd(PPh3)4 | DMF | 28 |
| 3 | CuI/Pd(PPh3)4 | Toluene | 16 |
| 4 | CuI/Pd(PPh3)4 | DMSO | 20 |
| 5e | CuI/Pd(OAc)2 | DMF | 51 |
| 6f | CuI/Pd(OAc)2 | DMF | 56 |
| 7g | CuI/Pd(OAc)2 | DMF | 76 |
| 8h | CuI/Pd(OAc)2 | DMF | 79 |
To extend the simplification of the reaction, by utilizing the above optimized conditions, the intramolecular C–H arylation of in situ generated 1,4-disubstituted triazoles was carried out using different aryl azides. As shown in Fig. 4, the coupling reaction proceeded well with almost all of the substrates and obtained good to excellent yields. All of the synthesized compounds were assessed for their in vitro anti proliferative activity against MCF-7, A-549 and HeLa cell lines. By comparing the results, it was clear that the electron withdrawing fluoro, chloro and nitro substituted aryl azides were found to have better reactivity than the electron donating methyl and methoxy substituted aryl azides (Fig. 4).
 | ||
| Fig. 4 Cu/Pd catalyzed one pot synthesis of benzoxazino[1,2,3]triazolyl[4,5-c]quinolinone. The isolated yields are given as percentages. | ||
In vitro cytotoxicity against human cancer cell lines
In vitro cytotoxic activity tests were carried out against human cell lines such as breast carcinoma (MCF-7), alveolar carcinoma (A-549) and cervical carcinoma (HeLa). Cisplatin was used as a positive control and the results are summarized in Table 2. Cell viability in the presence of the test samples was measured via the MTT-micro cultured tetrazolium assay.17 The relationship between the surviving fraction and drug concentration was plotted to obtain the survival curves of MCF-7 (Fig. 5), A-549 (Fig. 6) and HeLa (Fig. 7). The response parameter was expressed as an IC50 value, which corresponds to the concentration required for 50% inhibition of the cell viability. In vitro cytotoxic activity results revealed that the synthesized compounds showed significant to moderate activity against all the three tested cell lines. Interestingly, most of the analogues displayed potent activity against MCF-7. Among all of the compounds tested, compound 4d, having a 4-chloro-3,5-dimethoxyphenyl group on the triazole ring, exhibited potent activity against MCF-7 and A-549 cell lines with IC50 values of 11.18 ± 0.8 and 17.81 ± 0.6 μM and moderate activity against the HeLa cell line with an IC50 value of 35.16 ± 1.2 μM. These results are comparable to those of the standard drug cisplatin. Similarly, introduction of a 3,5-dimethyl group on the triazole skeleton, i.e.; 4b, significantly enhanced the cytotoxicity against MCF-7 and HeLa cell lines with IC50 values of 19.89 ± 0.8 and 21.69 ± 1.8 μM, and resulted in reasonable activity against the A-549 cell line with an IC50 value of 32.89 ± 1.1 μM, respectively. The compounds 4a and 4i bearing 4-methoxy phenyl and naphthyl substituents, respectively, on the triazole ring, exhibited good activity against the MCF-7 cell line with IC50 values of 22.75 ± 0.8 and 22.49 ± 1.6 μM, respectively. It is necessary to point out that all of the potent analogues contain electron donating substituents like 4-methoxyphenyl, 3,5-dimethtylphenyl, 4-chloro-3,5-dimethoxyphenyl and naphthyl on the triazole ring (4a, 4b, 4d & 4i) and this preference was uniform and irrespective to the substitution pattern on the aromatic ring. The electron withdrawing substituents such as chloro, fluoro, nitro and trifluoromethylphenyl groups on the triazole ring exhibited moderate to poor activity against all of the tested cell lines. Among all of the electron withdrawing substituents, compounds derived from 2-fluoro phenyl and 3-(trifluoromethyl)phenyl on the triazole ring (4k & 4e) showed reasonable activity against the MCF-7 cell line with IC50 values of 27.38 ± 1.5 and 31.18 ± 0.8 μM, respectively. The remaining compounds (4c, 4f, 4g, 4h and 4j) showed moderate to poor activity against the three cell lines with IC50 values ranging from 32.44 ± 1.3 to 112.72 ± 1.9 μM. On overall comparison, the compounds derived from electron donating substituents on the triazole ring exhibited potent activity and those with electron withdrawing substituents on the triazole ring exhibited moderate activity against the MCF-7 cell line, and all of the derivatives (4a–4k) exhibited good to moderate activity against A-549 and HeLa cell lines. Having obtained good activity for compounds 4a, 4b, 4d and 4i (IC50 < 23 μM) against cancer cell lines, we also evaluated their toxicity against a HEK-293 (Human Embryonic Kidney 293) cell line using MTT-micro cultured tetrazolium assay. The results are presented in Table 2.| Compound | MCF-7 | A-549 | HeLa | HEK293 |
|---|---|---|---|---|
| a Values are expressed as mean ± SEM. Cytotoxicity, as IC50, for each cell line is the concentration of compound which reduced the optical density of the treated cells by 50% with respect to the untreated cells using the MTT assay.b ND means not detected. | ||||
| 4a | 22.75 ± 0.8 | 41.02 ± 1.7 | 32.44 ± 1.3 | 40.89 ± 1.7 |
| 4b | 19.89 ± 0.8 | 32.89 ± 1.1 | 21.69 ± 1.8 | 34.22 ± 1.1 |
| 4c | 58.34 ± 1.1 | 51.58 ± 1.0 | 47.12 ± 1.4 | ND |
| 4d | 11.18 ± 0.8 | 17.81 ± 0.6 | 35.16 ± 1.2 | 29.08 ± 1.8 |
| 4e | 31.18 ± 0.8 | 67.81 ± 0.6 | 89.87 ± 1.6 | ND |
| 4f | 48.37 ± 1.2 | 43.24 ± 1.0 | 39.19 ± 1.7 | ND |
| 4g | 92.11 ± 1.3 | 112.72 ± 1.9 | 96.22 ± 1.4 | ND |
| 4h | 45.19 ± 1.3 | 52.42 ± 1.5 | 49.28 ± 1.8 | ND |
| 4i | 22.49 ± 1.6 | 36.58 ± 1.0 | 33.89 ± 1.7 | 44.58 ± 1.6 |
| 4j | 39.88 ± 1.9 | 41.34 ± 1.1 | 45.42 ± 1.8 | ND |
| 4k | 27.38 ± 1.5 | 36.89 ± 0.7 | 40.69 ± 1.2 | ND |
| Cisplatin | 4.61 ± 0.2 | 5.65 ± 0.2 | 3.86 ± 0.1 | ND |
Conclusion
In conclusion, a novel series of fused benzoxazino[1,2,3]triazolyl[4,5-c]quinolinone derivatives were synthesized via 3 + 2 cycloaddition followed by a palladium catalyzed in situ C–C bond coupling reaction without isolating the intermediate 1,4-disubstituted 1,2,3-triazole. All of the compounds were screened for their in vitro cytotoxic activity against MCF-7, A-549 and HeLa cancer cell lines. Compound 4d has shown a broad spectrum activity against MCF-7 and A-549. Compound 4b has shown prominent activity against MCF-7 and HeLa when compared with the standard drug, and it may be considered a as future drug candidate for cancer therapy. By affecting simple structural modifications in the titled compounds, new potent analogues for cancer therapy with good efficacy can be developed.Experimental
All of the reagents were of analytical grade or chemically pure. Analytical TLC was performed on silica gel 60 F254 plates. IR spectra (KBr pellet) were recorded on a Perkin-Elmer BX series FT-IR spectrometer. 1H NMR spectra were recorded on a Varian Gemini 400 MHz spectrometer. 13C NMR spectra were recorded on a Bruker 100 MHz spectrometer. Chemical shift values are given in ppm (δ) with tetramethylsilane as an internal standard. Mass spectral measurements were carried out using the EI method. Elemental analyses were performed on Carlo Erba 106 and Perkin-Elmer model 240 analyzers.General procedure for the synthesis of 5-iodo-4-(prop-2-yn-1-yl)-2H-benzo[b][1,4]oxazin-3(4H)-one (3)
To a mixture of 5-iodo-2H-benzo[b][1,4]oxazin-3(4H)-one (0.018 mol) and Cs2CO3 (0.054 mol) in acetone (50 mL), propargyl bromide (0.02 mol) was added at room temperature and stirred for 1 h. After completion of the reaction as shown by TLC analysis, the resulting mixture was concentrated under vacuum to afford a crude product. The crude product was diluted with cold water (50 mL) and stirred for 1 h. The resulting precipitate was collected and the crude product was purified via silica gel chromatography using an eluent (10% ethyl acetate in hexane). Yield: 89%; 1H NMR (400 MHz, CDCl3): δ 7.22–7.15 (m, 2H), 7.10–7.00 (m, 1H), 4.70–4.55 (m, 4H, O–CH2 & N–CH2), 2.25 (t, J = 2.6 Hz, 1H); 13C NMR (100 MHz, DMSO): 162.28, 137.50, 129.92, 126.65, 123.07, 112.58, 108.39, 73.89, 72.96, 67.45, 32.77; MS (ESI) m/z: 314 [M + H]+; anal. calcd for C11H8INO2: C, 42.20; H, 2.58; N, 4.47. Found: C, 42.26; H, 2.53; N, 4.41.General procedure for the synthesis of fused benzoxazino[1,2,3]triazolyl[4,5-c]quinolinone derivatives (4a–4k)
To a mixture of 5-iodo-4-(prop-2-yn-1-yl)-2H-benzo[b][1,4]oxazin-3(4H)-one 3 (1 mmol), aryl azide (1 mmol) and tBuOK (1 mmol) in dry N,N-dimethylformamide (10 mL) was added copper iodide (10 mol%) and Pd(OAc)2 (10 mol%), and the reaction mixture was stirred for 24–30 hours at 60–80 °C. After completion of the reaction as shown by TLC analysis, the reaction mixture was poured out on ice cooled water and extracted with ethyl acetate (50 mL). The combined organic layers were washed with water (2 × 20 mL) and brine (1 × 20 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified via column chromatography (silica gel, 25–30% ethyl acetate in hexane) to afford fused benzoxazino[1,2,3]triazolyl[4,5-c]quinolinones in good yields.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1578 (C
O), 1578 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1459, 1402 (N
C), 1459, 1402 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, CDCl3): δ 7.60 (d, J = 8.8 Hz, 2H), 7.52 (d, J = 8.8 Hz, 1H), 7.22–7.10 (m, 2H), 7.00 (d, J = 8.8 Hz, 2H), 5.20 (s, 2H, N–CH2), 4.65 (s, 2H, O–CH2), 3.89 (s, 3H, O–CH3); 13C NMR (100 MHz, DMSO): δ 164.26, 159.78, 146.33, 143.59, 130.42, 128.52, 125.80, 122.17, 119.83, 117.99, 115.35, 67.62, 56.07, 36.81; MS (ESI) m/z: 335 [M + H]+; anal. calcd for C18H14N4O3: C, 64.66; H, 4.22; N, 16.76. Found: C, 64.62; H, 4.27; N, 16.79.
N); 1H NMR (400 MHz, CDCl3): δ 7.60 (d, J = 8.8 Hz, 2H), 7.52 (d, J = 8.8 Hz, 1H), 7.22–7.10 (m, 2H), 7.00 (d, J = 8.8 Hz, 2H), 5.20 (s, 2H, N–CH2), 4.65 (s, 2H, O–CH2), 3.89 (s, 3H, O–CH3); 13C NMR (100 MHz, DMSO): δ 164.26, 159.78, 146.33, 143.59, 130.42, 128.52, 125.80, 122.17, 119.83, 117.99, 115.35, 67.62, 56.07, 36.81; MS (ESI) m/z: 335 [M + H]+; anal. calcd for C18H14N4O3: C, 64.66; H, 4.22; N, 16.76. Found: C, 64.62; H, 4.27; N, 16.79.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1593 (C
O), 1593 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1456, 1400 (N
C), 1456, 1400 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, CDCl3): δ 7.54–7.48 (m, 2H), 7.38 (s, 1H), 7.34–7.24 (m, 2H), 7.17 (s, 1H), 5.25 (s, 2H, N–CH2), 4.75 (s, 2H, O–CH2), 2.50 (s, 6H, Ar-CH3); 13C NMR (100 MHz, DMSO): δ 164.27, 146.34, 143.74, 139.87, 136.90, 130.49, 128.50, 125.81, 122.11, 119.84, 118.06, 117.92, 115.36, 67.63, 36.86, 21.32; MS (ESI) m/z: 333 [M + H]+; anal. calcd for C19H16N4O2: C, 68.66; H, 4.85; N, 16.86. Found: C, 68.74; H, 4.78; N, 16.81.
N); 1H NMR (400 MHz, CDCl3): δ 7.54–7.48 (m, 2H), 7.38 (s, 1H), 7.34–7.24 (m, 2H), 7.17 (s, 1H), 5.25 (s, 2H, N–CH2), 4.75 (s, 2H, O–CH2), 2.50 (s, 6H, Ar-CH3); 13C NMR (100 MHz, DMSO): δ 164.27, 146.34, 143.74, 139.87, 136.90, 130.49, 128.50, 125.81, 122.11, 119.84, 118.06, 117.92, 115.36, 67.63, 36.86, 21.32; MS (ESI) m/z: 333 [M + H]+; anal. calcd for C19H16N4O2: C, 68.66; H, 4.85; N, 16.86. Found: C, 68.74; H, 4.78; N, 16.81.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589 (C
O), 1589 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1469, 1403 (N
C), 1469, 1403 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, CDCl3): δ 7.70 (s, 2H), 7.50–7.35 (m, 2H), 7.22–7.07 (m, 2H), 5.21 (s, 2H, N–CH2), 4.62 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): δ 164.22, 144.27, 138.54, 135.73, 128.55, 128.38, 125.83, 122.64, 119.86, 119.10, 117.89, 115.42, 67.60, 36.76; MS (ESI) m/z: 374 [M + H]+; anal. calcd for C17H10Cl2N4O2: C, 54.71; H, 2.70; N, 15.01. Found: C, 54.77; H, 2.67; N, 14.95.
N); 1H NMR (400 MHz, CDCl3): δ 7.70 (s, 2H), 7.50–7.35 (m, 2H), 7.22–7.07 (m, 2H), 5.21 (s, 2H, N–CH2), 4.62 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): δ 164.22, 144.27, 138.54, 135.73, 128.55, 128.38, 125.83, 122.64, 119.86, 119.10, 117.89, 115.42, 67.60, 36.76; MS (ESI) m/z: 374 [M + H]+; anal. calcd for C17H10Cl2N4O2: C, 54.71; H, 2.70; N, 15.01. Found: C, 54.77; H, 2.67; N, 14.95.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1587 (C
O), 1587 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1472, 1411 (N
C), 1472, 1411 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, CDCl3): δ 7.60–7.42 (m, 3H), 7.25–7.08 (m, 3H), 5.25 (s, 2H, N–CH2), 4.63 (s, 2H, O–CH2), 3.90 (s, 3H, O–CH3), 3.83 (s, 3H, O–CH3); 13C NMR (100 MHz, DMSO): δ 164.29, 149.12, 146.40, 145.90, 142.47, 128.50, 126.26, 125.76, 125.01, 122.88, 119.87, 118.13, 115.72, 115.39, 110.75, 67.64, 57.51, 57.32, 36.46; MS (ESI) m/z: 399 [M + H]+; anal. calcd for C19H15ClN4O4: C, 57.22; H, 3.79; N, 14.05. Found: C, 57.16; H, 3.72; N, 14.12.
N); 1H NMR (400 MHz, CDCl3): δ 7.60–7.42 (m, 3H), 7.25–7.08 (m, 3H), 5.25 (s, 2H, N–CH2), 4.63 (s, 2H, O–CH2), 3.90 (s, 3H, O–CH3), 3.83 (s, 3H, O–CH3); 13C NMR (100 MHz, DMSO): δ 164.29, 149.12, 146.40, 145.90, 142.47, 128.50, 126.26, 125.76, 125.01, 122.88, 119.87, 118.13, 115.72, 115.39, 110.75, 67.64, 57.51, 57.32, 36.46; MS (ESI) m/z: 399 [M + H]+; anal. calcd for C19H15ClN4O4: C, 57.22; H, 3.79; N, 14.05. Found: C, 57.16; H, 3.72; N, 14.12.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1576 (C
O), 1576 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1465, 1409 (N
C), 1465, 1409 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, CDCl3): δ 7.99 (s, 1H), 7.94 (d, J = 7.9 Hz, 1H), 7.73–7.64 (m, 2H), 7.48 (d, J = 8.6 Hz, 1H), 7.22–7.17 (m, 1H), 7.14 (d, J = 2.1 Hz, 1H), 5.25 (s, 2H, N–CH2), 4.63 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): δ 164.24, 146.32, 144.19, 137.45, 131.80, 131.51, 131.19, 130.86, 130.54, 128.45, 125.83, 125.43, 124.45, 122.66, 119.86, 117.95, 117.19, 115.42, 67.62, 36.78; MS (ESI) m/z: 373 [M + H]+; anal. calcd for C18H11F3N4O2: C, 58.07; H, 2.98; N, 15.05. Found: C, 58.12; H, 2.92; N, 14.98.
N); 1H NMR (400 MHz, CDCl3): δ 7.99 (s, 1H), 7.94 (d, J = 7.9 Hz, 1H), 7.73–7.64 (m, 2H), 7.48 (d, J = 8.6 Hz, 1H), 7.22–7.17 (m, 1H), 7.14 (d, J = 2.1 Hz, 1H), 5.25 (s, 2H, N–CH2), 4.63 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): δ 164.24, 146.32, 144.19, 137.45, 131.80, 131.51, 131.19, 130.86, 130.54, 128.45, 125.83, 125.43, 124.45, 122.66, 119.86, 117.95, 117.19, 115.42, 67.62, 36.78; MS (ESI) m/z: 373 [M + H]+; anal. calcd for C18H11F3N4O2: C, 58.07; H, 2.98; N, 15.05. Found: C, 58.12; H, 2.92; N, 14.98.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1594 (C
O), 1594 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1467, 1413 (N
C), 1467, 1413 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, CDCl3): δ 7.78 (s, 1H), 7.70–7.60 (m, 1H), 7.55–7.38 (m, 3H), 7.22–7.10 (m, 2H), 5.23 (s, 2H, N–CH2), 4.65 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): δ 164.24, 146.31, 144.08, 138.00, 134.70, 132.11, 129.02, 128.44, 125.82, 122.46, 119.85, 119.07, 117.93, 115.40, 67.61, 36.79; MS (ESI) m/z: 339 [M + H]+; anal. calcd for C17H11ClN4O2: C, 60.28; H, 3.27; N, 16.54. Found: C, 60.34; H, 3.22; N, 16.61.
N); 1H NMR (400 MHz, CDCl3): δ 7.78 (s, 1H), 7.70–7.60 (m, 1H), 7.55–7.38 (m, 3H), 7.22–7.10 (m, 2H), 5.23 (s, 2H, N–CH2), 4.65 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): δ 164.24, 146.31, 144.08, 138.00, 134.70, 132.11, 129.02, 128.44, 125.82, 122.46, 119.85, 119.07, 117.93, 115.40, 67.61, 36.79; MS (ESI) m/z: 339 [M + H]+; anal. calcd for C17H11ClN4O2: C, 60.28; H, 3.27; N, 16.54. Found: C, 60.34; H, 3.22; N, 16.61.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1593 (C
O), 1593 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1459, 1400 (N
C), 1459, 1400 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, CDCl3): δ 8.32 (d, J = 7.9 Hz, 2H), 8.25–8.08 (m, 2H), 7.75 (t, J = 8.1 Hz, 1H), 7.45 (J = 8.5 Hz, 1H), 7.24–7.10 (m, 2H), 5.25 (s, 2H, N–CH2), 4.66 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): δ 164.27, 149.01, 146.31, 144.35, 137.56, 132.03, 128.44, 126.55, 125.84, 123.68, 122.79, 119.87, 117.93, 115.43, 115.20, 67.63, 36.78; MS (ESI) m/z: 350 [M + H]+; anal. calcd for C17H11N5O4: C, 58.45; H, 3.17; N, 20.05. Found: C, 58.41; H, 3.22; N, 20.12.
N); 1H NMR (400 MHz, CDCl3): δ 8.32 (d, J = 7.9 Hz, 2H), 8.25–8.08 (m, 2H), 7.75 (t, J = 8.1 Hz, 1H), 7.45 (J = 8.5 Hz, 1H), 7.24–7.10 (m, 2H), 5.25 (s, 2H, N–CH2), 4.66 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): δ 164.27, 149.01, 146.31, 144.35, 137.56, 132.03, 128.44, 126.55, 125.84, 123.68, 122.79, 119.87, 117.93, 115.43, 115.20, 67.63, 36.78; MS (ESI) m/z: 350 [M + H]+; anal. calcd for C17H11N5O4: C, 58.45; H, 3.17; N, 20.05. Found: C, 58.41; H, 3.22; N, 20.12.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589 (C
O), 1589 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1463, 1408 (N
C), 1463, 1408 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, CDCl3): δ 7.80–7.60 (m, 3H), 7.50–7.35 (m, 2H), 7.30–7.12 (m, 2H), 5.28 (s, 2H, N–CH2), 4.67 (s, 2H, O–CH2), 2.74 (t, J = 7.7 Hz, 2H), 1.73–1.58 (m, 2H), 1.51–1.34 (m, 2H), 1.01 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, DMSO): δ 164.26, 146.33, 143.66, 134.92, 130.06, 128.50, 125.79, 122.18, 120.45, 119.83, 117.98, 115.37, 67.63, 36.81, 34.75, 33.45, 22.20, 14.26; MS (ESI) m/z: 361 [M + H]+; anal. calcd for C21H20N4O2: C, 69.98; H, 5.59; N, 15.55. Found: C, 70.05; H, 5.63; N, 15.51.
N); 1H NMR (400 MHz, CDCl3): δ 7.80–7.60 (m, 3H), 7.50–7.35 (m, 2H), 7.30–7.12 (m, 2H), 5.28 (s, 2H, N–CH2), 4.67 (s, 2H, O–CH2), 2.74 (t, J = 7.7 Hz, 2H), 1.73–1.58 (m, 2H), 1.51–1.34 (m, 2H), 1.01 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, DMSO): δ 164.26, 146.33, 143.66, 134.92, 130.06, 128.50, 125.79, 122.18, 120.45, 119.83, 117.98, 115.37, 67.63, 36.81, 34.75, 33.45, 22.20, 14.26; MS (ESI) m/z: 361 [M + H]+; anal. calcd for C21H20N4O2: C, 69.98; H, 5.59; N, 15.55. Found: C, 70.05; H, 5.63; N, 15.51.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1598 (C
O), 1598 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1466, 1410 (N
C), 1466, 1410 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, CDCl3): δ 8.23–7.82 (m, 3H), 7.63–7.49 (m, 5H), 7.32–7.08 (m, 2H), 5.31 (s, 2H, N–CH2), 4.62 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): δ 164.32, 146.41, 142.93, 130.82, 128.87, 128.56, 128.48, 128.37, 127.62, 126.90, 125.91, 125.79, 124.41, 122.36, 119.85, 118.12, 115.38, 67.66, 36.81; MS (ESI) m/z: 355 [M + H]+; anal. calcd for C21H14N4O2: C, 71.18; H, 3.98; N, 15.81. Found: C, 71.29; H, 3.91; N, 15.74.
N); 1H NMR (400 MHz, CDCl3): δ 8.23–7.82 (m, 3H), 7.63–7.49 (m, 5H), 7.32–7.08 (m, 2H), 5.31 (s, 2H, N–CH2), 4.62 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): δ 164.32, 146.41, 142.93, 130.82, 128.87, 128.56, 128.48, 128.37, 127.62, 126.90, 125.91, 125.79, 124.41, 122.36, 119.85, 118.12, 115.38, 67.66, 36.81; MS (ESI) m/z: 355 [M + H]+; anal. calcd for C21H14N4O2: C, 71.18; H, 3.98; N, 15.81. Found: C, 71.29; H, 3.91; N, 15.74.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1594 (C
O), 1594 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1470, 1401 (N
C), 1470, 1401 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, CDCl3): δ 7.74–7.55 (m, 4H), 7.49 (d, J = 8.6 Hz, 1H), 7.24–7.10 (m, 2H), 5.22 (s, 2H, N–CH2), 4.62 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): 164.70, 146.52, 143.08, 139.45, 137.57, 136.49, 130.12, 126.72, 124.88, 123.02, 122.14, 120.29, 117.80, 67.92, 36.57; MS (ESI) m/z: 339 [M + H]+; anal. calcd for C17H11ClN4O2: C, 60.28; H, 3.27; N, 16.54. Found: C, 60.19; H, 3.21; N, 16.44.
N); 1H NMR (400 MHz, CDCl3): δ 7.74–7.55 (m, 4H), 7.49 (d, J = 8.6 Hz, 1H), 7.24–7.10 (m, 2H), 5.22 (s, 2H, N–CH2), 4.62 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): 164.70, 146.52, 143.08, 139.45, 137.57, 136.49, 130.12, 126.72, 124.88, 123.02, 122.14, 120.29, 117.80, 67.92, 36.57; MS (ESI) m/z: 339 [M + H]+; anal. calcd for C17H11ClN4O2: C, 60.28; H, 3.27; N, 16.54. Found: C, 60.19; H, 3.21; N, 16.44.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1573 (C
O), 1573 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1443, 1400 (N
C), 1443, 1400 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, CDCl3): δ 8.41 (d, J = 9.0 Hz, 1H), 8.05–7.91 (m, 1H), 7.74–7.61 (m, 1H), 7.55–7.41 (t, J = 7.5 Hz, 1H), 7.24–7.10 (m, 3H), 5.23 (s, 2H, N–CH2), 4.63 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): 164.34, 148.76, 146.69, 141.71, 138.19, 137.21, 131.91, 130.88, 125.91, 126.42, 124.52, 123.68, 121.80, 119.94, 117.68, 67.70, 36.62; MS (ESI) m/z: 323 [M + H]+; anal. calcd for C17H11FN4O2: C, 63.35; H, 3.44; N, 17.38. Found: C, 63.44; H, 3.37; N, 17.47.
N); 1H NMR (400 MHz, CDCl3): δ 8.41 (d, J = 9.0 Hz, 1H), 8.05–7.91 (m, 1H), 7.74–7.61 (m, 1H), 7.55–7.41 (t, J = 7.5 Hz, 1H), 7.24–7.10 (m, 3H), 5.23 (s, 2H, N–CH2), 4.63 (s, 2H, O–CH2); 13C NMR (100 MHz, DMSO): 164.34, 148.76, 146.69, 141.71, 138.19, 137.21, 131.91, 130.88, 125.91, 126.42, 124.52, 123.68, 121.80, 119.94, 117.68, 67.70, 36.62; MS (ESI) m/z: 323 [M + H]+; anal. calcd for C17H11FN4O2: C, 63.35; H, 3.44; N, 17.38. Found: C, 63.44; H, 3.37; N, 17.47.Cytotoxic activity
All of the synthesized compounds were evaluated for their in vitro cytotoxic activity against three different cancer cell lines such as MCF-7 (breast), A-549 (alveolar) and HeLa (cervical). All of the cancer cell lines used in this research work were obtained from National Centre for Cell Sciences (NCCS), Pune, India. Cell viability in the presence of the test samples was measured using the MTT-microcultured tetrazolium assay. This assay is a quantitative colorimetric method for the determination of cell cytotoxicity. The assessed parameter is the metabolic activity of viable cells. Metabolically active cells reduce pale yellow tetrazolium salt (MTT) to a dark blue water-insoluble formazan, which can be directly quantified after solubilization with DMSO. The absorbance of the formazan directly correlates with the number of viable cells. Human cells were plated into a 96-well plate at a density of 1 × 104 cells per well. Cells were grown overnight in the full medium and then switched to the low serum media. DMSO was used as a control. After 48 h of treatment with different concentrations of test compounds, the cells were incubated with MTT (2.5 mg mL−1) in the CO2 chamber for 2 h. The medium was then removed and 100 μL of DMSO was added into each well to dissolve the formazan crystals. After thoroughly mixing, the plates were read at 570 nm for optical density which is directly correlated with cell quantity. The results were represented as a percentage of cytotoxicity/viability. All of the experiments were carried out in triplicates. The IC50 values were calculated from the percentage of cytotoxicity and compared with the reference drug cisplatin (Table 2).Determination of the IC50 values
The IC50 values were determined from plots of the dose response curve between compound concentration and % of cell viability. The IC50 values were derived using curve fitting methods with Graph Pad Prism as statistical software (Ver. 5.02). The average of three runs (triplicate manner) was taken in the determination. The graphs were plotted by keeping the concentration of drug on the X-axis and % of cell viability on the Y-axis. The dose–response profiles for the compounds on human MCF-7, A-549 and HeLa cell lines are shown in Fig. 5–7. The IC50 values of the compounds against three different cell lines were calculated by plotting a graph of % of cell viability versus compound concentration.Acknowledgements
We thank the University Grants Commission, New Delhi, India for the financial support. Narsimha and Satheesh Kumar thank CSIR New Delhi, for the award of senior research fellowships.References
-
(a) H. Nandivada, X. Jiang and P. Lahann, Adv. Mater., 2007, 19, 2197 CrossRef CAS
; (b) Y. L. Angell and K. Burgess, Chem. Soc. Rev., 2007, 36, 1674 RSC
; (c) D. Fournier, R. Hoogenboom and U. S. Schubert, Chem. Soc. Rev., 2007, 36, 1369 RSC
; (d) J. E. Moses and A. D. Moorhouse, Chem. Soc. Rev., 2007, 36, 1249 RSC
; (e) M. J. Giffin, H. Heaslet, A. Brik, Y.-C. Lin, G. Cauvi, C.-H. Wong, D. E. McRee, J. H. Elder, C. D. Stout and B. E. Torbett, J. Med. Chem., 2008, 51, 6263 CrossRef CAS PubMed
; (f) K. D. Hänni and D. A. Leigh, Chem. Soc. Rev., 2010, 39, 1240 RSC
; (g) J.-F. Lutz, Angew. Chem., Int. Ed., 2007, 46, 1018 CrossRef CAS PubMed
; (h) A. Dondoni, Chem.–Asian J., 2007, 2, 700 CrossRef CAS PubMed
; (i) H. C. Kolb and K. B. Sharpless, Drug Discovery Today, 2003, 8, 1128 CrossRef CAS PubMed
; (j) R. Alvarez, S. Velazquez, A. San-Felix, S. Aquaro, E. De Clercq, C.-F. Perno, A. Karlsson, J. Balzarini and M. J. Camarasa, J. Med. Chem., 1994, 37, 4185 CrossRef CAS PubMed
; (k) P. W. Baures, Org. Lett., 1999, 1, 249 CrossRef CAS PubMed
; (l) S. G. Agalave, S. R. Maujan and V. S. Pore, Chem.–Asian J., 2011, 6, 2696 CrossRef CAS PubMed
; (m) T. Jin, S. Kamijo and Y. Yamamoto, Eur. J. Org. Chem., 2004, 18, 3789 CrossRef
; (n) T. Müller and S. Bräse, Angew. Chem., Int. Ed., 2011, 50, 11844 CrossRef PubMed
.
-
(a) A. C. Tome, Sci. Synth., 2004, 13, 415 CAS
; (b) A. Tam, U. Arnold, M. B. Soellner and R. T. Raines, J. Am. Chem. Soc., 2007, 129, 12670 CrossRef CAS PubMed
; (c) S. Ito, A. Satoh, Y. Nagatomi, Y. Hirata, G. Suzuki, T. Kimura, A. Satow, S. Maehara, H. Hikichi, M. Hata, H. Kawamoto and H. Ohta, Bioorg. Med. Chem., 2008, 16, 9817 CrossRef CAS PubMed
; (d) S. Ito, Y. Hirata, Y. Nagatomi, A. Satoh, G. Suzuki, T. Kimura, A. Satow, S. Maehara, H. Hikichi, M. Hata, H. Ohta and H. Kawamoto, Bioorg. Med. Chem. Lett., 2009, 19, 5310 CrossRef CAS PubMed
; (e) J. M. Holub and K. Kirshenbaum, Chem. Soc. Rev., 2010, 39, 1325 RSC
; (f) M. Fujinaga, T. Yamasaki, K. Kawamura, K. Kumata, A. Hatori, J. Yui, K. Yanamoto, Y. Yoshida, M. Ogawa, N. Nengaki, J. Maeda, T. Fukumura and M.-R. Zhang, Bioorg. Med. Chem., 2011, 19, 102 CrossRef CAS PubMed
; (g) I. E. Valverde, A. Bauman, C. A. Kluba, S. Vomstein, M. A. Walter and T. L. Mindt, Angew. Chem., Int. Ed., 2013, 52, 8957 CrossRef CAS PubMed
; (h) S. Li and Y. Huang, Curr. Med. Chem., 2014, 21, 113 CrossRef CAS PubMed
.
- I. S. Bennet, G. Brooks, N. J. P. Broom, S. H. Calvert, K. Coleman and I. Francois, J. Antibiot., 1991, 44, 969 CrossRef
.
- G. A. Stilwell, H. G. Adams and M. Turck, Antimicrob. Agents Chemother., 1975, 8, 751 CrossRef CAS PubMed
.
- M. J. Soltis, H. J. Yeh, K. A. Cole, N. Whittaker, R. P. Wersto and E. C. Kohn, Drug Metab. Dispos., 1996, 24, 799 CAS
.
-
(a) H. M. Niemeyer, J. Agric. Food Chem., 2009, 57, 1677 CrossRef CAS PubMed
; (b) M. Packiarajan, C. G. Mazza Ferreira, S. P. Hong, A. D. White, G. Chandrasena, X. Pu, R. M. Brodbeck and A. J. Robichaud, Bioorg. Med. Chem. Lett., 2012, 22, 5658 CrossRef CAS PubMed
; (c) L. Wu, K. Lu, M. Packiarajan, V. Jubian, G. Chandrasena, T. C. Wolinsky and M. W. Walker, Bioorg. Med. Chem. Lett., 2012, 22, 2167 CrossRef CAS PubMed
; (d) W. H. Wu, T. Y. Chen, R. W. Lu, S. T. Chen and C. C. Chang, Phytochemistry, 2012, 83, 110 CrossRef CAS PubMed
.
- P. S. Filippou, E. N. Koini, T. Calogeropoulou, P. Kalliakmani, C. A. Panagiotidis and D. A. Kyriakidis, Bioorg. Med. Chem., 2011, 19, 5061 CrossRef CAS PubMed
.
-
(a) R. Fringuelli, D. Pietrella, F. Schiaffella, A. Guarraci, S. Perito, F. Bistoni and A. Vecchiarelli, Bioorg. Med. Chem., 2002, 10, 1681 CrossRef CAS PubMed
; (b) R. Fringuelli, N. Giacche, L. Milanese, E. Cenci, A. Macchiarulo, A. Vecchiarelli, G. Costantino and F. Schiaffella, Bioorg. Med. Chem., 2009, 17, 3838 CrossRef CAS PubMed
.
-
(a) C. Rajitha, P. K. Dubey, S. Venkataiah, P. F. Javier, R. V. Venugopal and P. Manojit, Eur. J. Med. Chem., 2011, 46, 4887 CrossRef CAS PubMed
; (b) Z. Xue-Wen, M. Han-Lin, Z. Xuan, J. Shi-Yao, M. Jun-Ying and Z. Bao-Xiang, Eur. J. Med. Chem., 2014, 79, 95 CrossRef PubMed
.
- P. Zhong-Tai, G. Li-Ping, Z. Li-Ming, P. Hu-Ri and Q. Zhe-Shan, Eur. J. Med. Chem., 2008, 43, 1216 CrossRef PubMed
.
-
(a) A. N. Matralis, M. G. Katselou, A. Nikitakis and A. P. Kourounakis, J. Med. Chem., 2011, 54, 5583 CrossRef CAS PubMed
; (b) T. Hasui, N. Matsunaga, T. Ora, N. Ohyabu, N. Nishigaki, Y. Imura, Y. Igata, H. Matsui, T. Motoyaji, T. Tanaka, N. Habuka, S. Sogabe, M. Ono, C. S. Siedem, T. P. Tang, C. Gauthier, L. A. De Meese, S. A. Boyd and S. Fukumoto, J. Med. Chem., 2011, 54, 8616 CrossRef CAS PubMed
.
-
(a) S. Chuprakov, N. Chernyak, A. S. Dudnik and V. Gevorgyan, Org. Lett., 2007, 9, 2333 CrossRef CAS PubMed
; (b) L. Ackermann, R. Vicente and R. Born, Adv. Synth. Catal., 2008, 350, 741 CrossRef CAS
; (c) L. Ackermann, A. Althammer and S. Fenner, Angew. Chem., Int. Ed., 2009, 48, 201 CrossRef CAS PubMed
; (d) L. Ackermann and R. Vicente, Org. Lett., 2009, 11, 4922 CrossRef CAS PubMed
; (e) L. Ackermann, H. K. Potukuchi, D. Landsberg and R. Vicente, Org. Lett., 2008, 10, 3081 CrossRef CAS PubMed
; (f) P. Xi, F. Yang, S. Qin, D. Zhao, J. Lan, G. Gao, C. Hu and J. You, J. Am. Chem. Soc., 2010, 132, 1822 CrossRef CAS PubMed
; (g) Z. Liang, J. hao and Y. Zhang, J. Org. Chem., 2010, 75, 170 CrossRef CAS PubMed
; (h) K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2009, 131, 9651 CrossRef CAS PubMed
; (i) T. Watanabe, S. Oishi, N. Fujii and H. Ohno, J. Org. Chem., 2009, 74, 4720 CrossRef CAS PubMed
; (j) S. H. Cho, S. J. Hwang and S. Chang, J. Am. Chem. Soc., 2008, 130, 9254 CrossRef CAS PubMed
; (k) H. Jiang, Z. Feng, A. Wang, X. Liu and Z. Chen, Eur. J. Org. Chem., 2010, 1227 CrossRef
; (l) M. D. K. Boele, G. P. F. van Strijdonck, A. H. M. de Vries, P. C. J. Kamer, J. G. de Vries and P. W. N. M. van Leeuwen, J. Am. Chem. Soc., 2002, 124, 1586 CrossRef CAS PubMed
.
- C.-Y. Chen, C.-H. Yang, W.-P. Hu, J. K. Vandavasi, M.-I. Chung and J.-J. Wang, RSC Adv., 2013, 3, 2710 RSC
.
- J. Panteleev, K. Geyer, A.-A. Angelica, L. Wang and M. Lautens, Org. Lett., 2010, 12, 5092 CrossRef CAS PubMed
.
- R. Jeyachandran, H. K. Potukuchi and L. Ackermann, Beilstein J. Org. Chem., 2012, 8, 1771 CrossRef CAS PubMed
.
-
(a) S. Narsimha, T. R. Kumar, N. S. Kumar, S. Yakub and N. V. Reddy, Med. Chem. Res., 2014, 23, 5321 CrossRef CAS
; (b) S. Narsimha, N. S. Kumar, B. K. Swamy, N. V. Reddy, S. K. Althaf Hussain and M. Srinivasa Rao, Bioorg. Med. Chem. Lett., 2016, 26, 1639 CrossRef CAS PubMed
; (c) K. Battula, S. Narsimha, V. Nagavelli, P. Bollepelli and M. Srinivasa Rao, J. Serb. Chem. Soc., 2015, 81, 233 CrossRef
; (d) S. Narsimha, B. K. Swamy, L. Sudhakar, N. V. Reddy and S. K. Althaf Hussain, Phosphorus, Sulfur Silicon Relat. Elem., 2016, 191, 1118 CrossRef
.
-
(a) F. Denizot and R. Lang, J. Immunol. Methods, 1986, 89, 271 CrossRef CAS PubMed
; (b) J. A. Kumar, G. Saidachary, G. Mallesham, B. Sridhar, N. Jain, S. V. Kalivendi, V. J. Rao and B. C. Raju, Eur. J. Med. Chem., 2013, 65, 389 CrossRef CAS PubMed
; (c) B. C. Raju, R. N. Rao, P. Suman, P. Yogeeswari, D. Sriram, T. B. Shaik and S. V. Kalivendi, Bioorg. Med. Chem. Lett., 2011, 21, 2855 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12285j |
| This journal is © The Royal Society of Chemistry 2016 |