Nitrogen-doped carbon dots derived from polyamindoamine dendrimer†
Juncai Shena,
Qing Lia,
Yan Zhanga,
Xing-jin Shea,
Cai-Feng Wanga and
Su Chen*ab
aState Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing Tech University (former: Nanjing University of Technology), Nanjing 210009, P. R. China. E-mail: chensu@njtech.edu.cn; Fax: +86-25-83172258; Tel: +86-25-83172258
bJiangsu Key Lab of Fine Chemical & Functional Polymer Materials, China
First published on 13th June 2016
Abstract
Nitrogen-doped carbon dots (CDs) were synthesized through a hydrothermal process by using N-riched polyamindoamine (PAMAM) dendrimer as the precursor. The CDs depict bright blue fluorescence with the quantum yield (QY) of 40%, and show excellent monodispersity and solubility in water. The CDs also demonstrate sensitive detection of Fe3+ ions (ppm level). For more practical applications, the as-prepared CDs could be ink candidates for nano-printing patterns, which are highly fluorescent. Also, we have found the pH values and solvents used in the ink have great influence on the intensity of their fluorescence. More interestingly, we prepared poly(N-isopropylacrylamide) (PNIPAM) and CDs hybrids, which exhibit temperature switching properties. They were reversibly sensitive to external temperatures ranging from 20 °C to 40 °C, making it possible to develop CD-based thermosensitive devices or sensors.
Introduction
Carbon dots (CDs), as an emerging nanomaterial, have attracted considerable attention since their discovery in 20041 due to a wide range of applications, including in bioimaging,2 in vivo theranostics,3,4 drug delivery,5 light-emitting diodes,6–8 photocatalysis9 and solar cells.10 Compared with traditional semiconductor quantum dots (QDs), CDs show many advantages, such as low toxicity, low cost, tuneable fluorescence emission, excellent photostability, up-conversion and biocompatibility.11,12 A variety of methods have been developed to prepare CDs, which can generally be classified into “top–down” and “bottom–up” approaches. The former methods involve plasma treatments,13 laser ablation,14,15 electrochemical synthesis,16 where the CDs are generated from larger carbonaceous materials, such as carbon nanotubes,17 carbon soot,18 activated carbon,19 coal20 and graphene oxide.21 The latter approaches involve hydrothermal, microwave and ultrasonic synthetic routes, where the CDs are produced from small molecule precursors such as citrate,22 ethylene glycol23 and urea.24Nevertheless, compared with conventional semiconductor QDs, most of the obtained CDs have a relatively low QY. Doping photoluminescent (PL) materials with heteroatoms can effectively tune surface and local chemical features, especially PL properties.25 Many elements, such as N,26 S,27,28 P,26,29 have been reported to improve the optical and electronic properties of CDs. Among these, nitrogen doping or using nitrogen containing precursors has been proved to be a very effective route for tailoring the properties of CDs, since N atom possesses comparable atomic size and five valence electrons for bonding with carbon atoms.30,31 Chen et al.32 used 2-azidoimidazole as the precursor, via a hydrothermal process at 70 °C overnight, and they prepared nitrogen-rich CDs with QY value as 33%. Yu et al.33 synthesized nitrogen-doped CDs from bee pollens and applied in bioimaging and catalysis. In another study, Ling et al.34 used the waste chicken eggshell to prepare nitrogen-doped CDs through a pyrolysis process at 300 °C. However, most of the nitrogen-doped CDs are unsatisfactory due to harsh synthetic conditions and long reaction time. Thus, the preparation of nitrogen-doped CDs with timesaving and eco-friendly process is a high concern.
Herein, we reported a facile and eco-friendly method to obtain bright blue fluorescence nitrogen-doped CDs with the QY of 40% (Scheme 1). The CDs depict excellent monodispersity, solubility in water and sensitivity detection of Fe3+ ions (ppm level). Also, the N-riched PAMAM dendrimer precursor makes it facile to synthesis of nitrogen-doped CDs without further passivation or dopant. To be more practical applications, the as-prepared CDs could be the ink candidates of nano-printing patterns, which are of high fluorescence. Also, we have found the pH values and solvents used in the ink have great influence on the intensity of their fluorescence. More interestingly, the fabricated CDs were doped into NIPAM solution, forming CDs/PNIPAM hybrids, which exhibit temperature switch property. The reversibly sensitive external temperatures from 20 °C to 40 °C, makes it possible to develop CDs-based thermo-sensitive devices or sensors. This strategy might provide an available pathway to handy access of CDs.
 | ||
| Scheme 1 Schematic illustration for the synthesis process of CDs and their multifunctional applications. | ||
Experimental
Materials
Polyamindoamine (PAMAM) dendrimer (G0) was purchased from Weihai CY Dendrimer Technology Co., Ltd. Citric acid anhydrous, polyvinylpyrrolidone (PVP) and N-isopropyl acrylamide were bought from Aladdin. Solvents including ethylene glycol (EG), N,N-dimethylformamide (DMF), toluene, acetone, ethanol, tetramethylethylenediamine (TMEDA) were of analytical reagent grade and purchased from Sinopharm Chemical Reagent Co., Ltd. N,N-Methylenebisacrylamide (MBAA), ammonium persulfate (APS), iron(III) chloride hexahydrate (FeCl3·6H2O), iron(II) sulfate heptahydrate (FeSO4·7H2O), copper(II) chloride dihydrate (CuCl2·2H2O), sodium chloride (NaCl), cadmium chloride hydrate (CdCl2·2.5H2O), chromic(III) chloride hexahydrate (CrCl3·6H2O), lead(II) acetate trihydrate (C4H6O4Pb·3H2O), cobalt(II) chloride hexahydrate (CoCl2·6H2O), zinc chloride (ZnCl2), calcium chloride anhydrous (CaCl2), aluminum nitrate nonahydrate [Al(NO3)3·9H2O], magnesium chloride hexahydrate (MgCl2·6H2O), barium chloride dihydrate (BaCl2·2H2O) and mercury(II) sulphate (HgSO4) were purchased from standard sources. All chemicals were used as received without further purification. High-purity water with resistivity greater than 18 MΩ cm−1 was used in all experiments.Preparation of CDs
CDs were prepared by the hydrothermal process. Typically, citric acid (0.5 g) and PAMAM dendrimer (20 wt% in water, 500 μL) were dissolved in 20 mL DI-water. After homogeneous mixing, the solution was transferred to a poly(tetrafluoroethylene) (Teflon)-lined autoclave (50 mL) and heated at 200 °C for 5 h. After the reaction, the autoclave was cooled to room temperature naturally. Then the product was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to remove larger particles. The resultant brown supernatant was further filtered with an ultra-filtration membrane (0.22 μm) to remove impurities. The filtrate was collected for further characterization and use.
000 rpm for 10 min to remove larger particles. The resultant brown supernatant was further filtered with an ultra-filtration membrane (0.22 μm) to remove impurities. The filtrate was collected for further characterization and use.
Preparation of CDs inks for fluorescent patterns
For silk-screen printing, 2.0 g polyvinylpyrrolidone (PVP) and 1.5 mg CDs solid were dissolved in 10.0 g different solvents (namely DMF, water and alcohol) and magnetically blended to form a homogeneous solution. Filter paper was chosen as printed paper, which is featured no background fluorescence under the UV lamp. The solution was cast on the printing mask of a silk screen printing device, and was penetrated through the “Tai Chi” pattern screen (160 mesh) onto the paper substrate. For nano-printing, 1.5 mg CDs solid was magnetically blended with 10.0 g water to form a homogeneous solution, which was then encapsulated into the ink cartridge of a Jetlab®II Precision Printing Platform for nano-printing at room temperature.Synthesis of PNIPAM/CDs hybrids
For PNIPAM hybrid, first of all, 0.5 g NIPAM was dissolved in 1.0 g EG in a beaker with magnetic stirring. Then, 0.005 g APS and 0.01 g MBAA were added and shaken vigorously to obtain a homogeneous mixture before 0.01 g TMEDA solution was added. Then the beaker was closed and kept polymerization for 2 h at room temperature. The resulting PNIPAM hybrid was washed with DI-water several times to remove the unreacted monomers. For PNIPAM/CDs hybrids, 0.5 g NIPAM and 0.01 g CDs were dissolved in 1.0 g EG. Others process were same as the preparation of PNIPAM hybrid.Detection of metal ions
Typically, 2.0 mL of 0.001 mol L−1 different metal ions solution was added into 2 mL of CDs stock solution (0.1 mg mL−1), and equilibrated for 10 min at room temperature before the PL spectral measurements. The PL measurements were recorded under excitation at 360 nm. For selectivity detection of Fe3+ ion, different concentrations of Fe3+ solution were added instead of different metal ions in a similar way.Characterization
PL spectra of the CDs were measured with a Varian Cary Eclipse fluorescence spectrophotometer at ambient conditions. Transmission electron microscope (TEM) was recorded on a JEOL JEM-2100 electron microscope. The X-ray diffraction (XRD) pattern was obtained on a Bruker AXS D8-ADVANCE X-ray diffractometer with Cu/Kα radiation over a 2θ range of 10–80°. Raman spectra were recorded using a Horiba HR 800 Raman system equipped with a 514.5 nm laser. Elemental analysis was performed on an Elementar Vario EL III. X-ray photoelectron spectroscopy (XPS) spectra of the CDs were performed on an ES-CAIAB250 XPS system with Al/Kα as the source. Fourier transform infrared (FT-IR) spectrum was taken on a Nicolet 6700 FT-IR spectrophotometer with the KBr powder with 32 scans from 4000 to 500 cm−1 at a resolution of 4 cm−1. Ultraviolet-visible (UV-vis) spectrum was obtained using a Perkin-Elmer Lambda 900 UV-vis spectrometer. Time-resolved fluorescence decay curve was attained on the leica SP5 FLIM system using 405 nm laser as the excitation source.Results and discussion
Structures of the CDs
The synthesis of blue fluorescent nitrogen-doped CDs started from a hydrothermal process at 200 °C for 5 h, which employed PAMAM dendrimer (N content 27%) and citric acid as the precursors. Elemental analysis shown in Table S1 (ESI†) reveals the nitrogen content of CDs is reached 6.99%, indicating N-doped CDs have been prepared. Also, the CDs solution shows bright blue fluorescence under UV light. The structures of the CDs were investigated by the transmission electron microscopy (TEM), as indicated in Fig. 1a. It reveals that the CDs appear as spherical particles and have uniform dispersion without apparent aggregation. The corresponding size distribution histogram (Fig. 1a, insert) confirms that the size of the CDs ranges from 2.2 to 3.4 nm with an average diameter of 2.8 nm. The X-ray diffraction (XRD) was used to detect the crystallinity of the CDs. Similar to the previously reported,35 the XRD pattern displays a broad (002) peak around 2θ = 22° (Fig. 1b), indicating the amorphous structure of the CDs.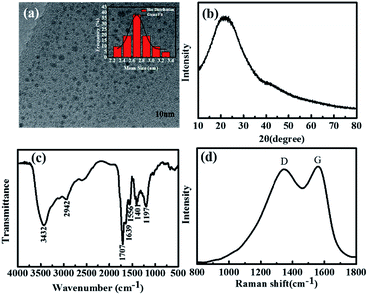 | ||
| Fig. 1 (a) TEM image of the CDs (insert shows the corresponding size histogram of the CDs). (b) XRD pattern, (c) FT-IR spectrum and (d) Raman spectrum of the CDs. | ||
FT-IR spectrum was used to determine the surface functional groups of the CDs. As shown in Fig. 1c, the obvious peak around 3432 cm−1 is attributed to the stretching vibrations of the –OH group. 2942 cm−1 peak can be assigned to the –CH2 vibrations. 1707 cm−1 peak refers to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, and 1556 cm−1 refers to the bending vibrations of N–H. The vibrational absorption at 1639 cm−1 peak belongs to C
O group, and 1556 cm−1 refers to the bending vibrations of N–H. The vibrational absorption at 1639 cm−1 peak belongs to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, and 1401 cm−1 peak originates from the C–N stretching vibrations. And 1197 cm−1 peak can be identified as C–O stretching. The Raman spectrum (Fig. 1d) of CDs shows obviously a D band at 1346 cm−1 and a G band at 1560 cm−1, indicating that the CDs mainly contain sp2 carbons with some sp3 hybrid carbons.36 The above results suggest that the as-prepared CDs are mainly composed of sp2 carbons with some sp3 hybrid carbons and abundant hydrophilic groups at their surfaces.
O stretching vibration, and 1401 cm−1 peak originates from the C–N stretching vibrations. And 1197 cm−1 peak can be identified as C–O stretching. The Raman spectrum (Fig. 1d) of CDs shows obviously a D band at 1346 cm−1 and a G band at 1560 cm−1, indicating that the CDs mainly contain sp2 carbons with some sp3 hybrid carbons.36 The above results suggest that the as-prepared CDs are mainly composed of sp2 carbons with some sp3 hybrid carbons and abundant hydrophilic groups at their surfaces.
XPS spectra of CDs
X-ray photoelectron spectroscopy (XPS) was used to further investigate the chemical structure of the CDs. The XPS spectrum in Fig. 2a reveals the existence of carbon (C 1s, 285 eV), nitrogen (N 1s, 400 eV) and oxygen (O 1s, 532 eV) at the surface of CDs, which is also consistent with the elemental analysis results. The high-resolution spectra of C 1s peaks at 284.6, 286.2, and 288.4 eV (Fig. 2b) correspond to sp2 carbons (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), sp3 carbons (C–O/C–N), carbonyl carbons (C
C), sp3 carbons (C–O/C–N), carbonyl carbons (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), respectively.34,37 The high-resolution N 1s spectra of the CDs in shown Fig. 2c reveal two types of nitrogen, i.e., amino N (399.5 eV) and pyrrolic N (400.1 eV), respectively.38 In addition, the O 1s spectra (Fig. 2d) contain two peaks at 531.3 and 532.3 eV, which can be attributed to C
O), respectively.34,37 The high-resolution N 1s spectra of the CDs in shown Fig. 2c reveal two types of nitrogen, i.e., amino N (399.5 eV) and pyrrolic N (400.1 eV), respectively.38 In addition, the O 1s spectra (Fig. 2d) contain two peaks at 531.3 and 532.3 eV, which can be attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O, respectively.39
O and C–O, respectively.39
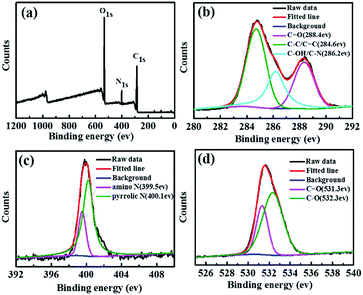 | ||
| Fig. 2 XPS spectra (a) and high-resolution XPS of C 1s (b), N 1s (c), and O 1s (d) spectra of the CDs. | ||
Fluorescent properties of CDs
The as-prepared nitrogen-doped CDs exhibit favourable photoluminescence properties. Fig. 3a demonstrates the UV-vis absorption spectrum and photoluminescence emission spectrum of the CDs. An obvious absorption peak at around 345 nm can be observed, which can be attributed to the n–π* transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.40 At 360 nm excitation, the photoluminescence peak is cantered at 460 nm. Inset picture in Fig. 3a shows the CDs aqueous solution under 365 nm UV light. Bright blue fluorescence can be observed by naked eyes, which also correspond to the PL emission spectrum excited at 360 nm. As show in Fig. 3b, excitation-dependent PL behaviour of the as-prepared CDs was detected.15 By varying the excitation wavelength from 320 nm to 460 nm, the maximum PL emission of the CDs red-shifts from 446 nm to 530 nm. The QY of the CDs is determined to be 40% by using quinine sulfate as a reference (Table S2, ESI†).
O.40 At 360 nm excitation, the photoluminescence peak is cantered at 460 nm. Inset picture in Fig. 3a shows the CDs aqueous solution under 365 nm UV light. Bright blue fluorescence can be observed by naked eyes, which also correspond to the PL emission spectrum excited at 360 nm. As show in Fig. 3b, excitation-dependent PL behaviour of the as-prepared CDs was detected.15 By varying the excitation wavelength from 320 nm to 460 nm, the maximum PL emission of the CDs red-shifts from 446 nm to 530 nm. The QY of the CDs is determined to be 40% by using quinine sulfate as a reference (Table S2, ESI†).
We further investigated the fluorescent stability of the CDs. Time-correlated single photon counting (TCSPC) was applied to measurement the fluorescence lifetime (τ) of the CDs. As presented in Fig. 3c, the decay lifetime of the CDs can be acquired from eqn (1):
Y(t) = α1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + α2 exp(−t/τ1) + α2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) exp(−t/τ2)
| (1) |
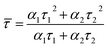 | (2) |
The calculated average lifetime for the CDs is 3.36 ± 0.05 ns (χ2 < 1.1) (Table S3, ESI†), which is similar to the reported values of other researchers.41 Moreover, the CDs demonstrate excellent photostability and resistance of photobleaching. No obvious PL intensity changes were observed after being stored for six months (Fig. 3d) and continuous UV illumination (∼1 W, 365 nm) for 2 h (Fig. S1, ESI†).
CDs inks for printing fluorescent patterns
We used the CDs as fluorescence ink for printing versatile fluorescent patterns. Fig. 4a shows the fluorescent Tai Chi patterns by silk-screen printing at different pH, which we can clearly distinguish the difference. The patterns depict bright blue fluorescence under neutral environment, while show weak fluorescence in acidic or basic conditions, especially in strong acid. We also apply CDs different solvents solution as fluorescence ink for silk-screen printing, as shown in Fig. 4b. The pattern printed from aqueous solution is the brightest, then from DMF solution, while the pattern printed from CDs ethanol solution appears to be the darkest. It seems CDs aqueous solution in neutral atmosphere is the best condition for silk-printing. So we further applied CDs aqueous solution at pH = 7 as fluorescence ink for printing via a nano-printing device (Fig. S2, ESI†). We could clearly catch sight of the blue fluorescent butterfly and lotus pattern, as shown in Fig. 4c. Compared with silk-screen printing, nano-printing patterns seem to be more legible and well-defined. The printed fluorescent patterns are very stable and showed bright blue fluorescence even after placing for two months (Fig. S3, ESI†). It should be pointed out that the CDs ink has potential application in preventing fraud and anti-counterfeit fields.In order to illustrate the excellent monodispersity and solubility in aqueous solution, we further investigate the effect of different pH values and various solvents on the PL spectra of the CDs. Fig. 4d depicts the effect of different pH values on PL intensity of the CDs. The PL intensity is nearly constant in the pH range of 6–11, and changes pronouncedly either in a strong acidic or a strong basic regions. With pH increasing from 1 to 6, we can observe nearly a linearly increase of the PL intensity. Nevertheless, with pH enhanced from 11 to 13, the PL intensity of CDs decreased sharply. The results correspond to the printed fluorescent patterns in Fig. 4a, since the PL intensity in pH = 2 is lower than in pH = 12, and the PL intensity in pH = 7 is the highest. The deprotonation of oxygen containing functional groups, such as carboxyl groups, may account for the differences PL intensity.42,43 Another interesting phenomenon is solvent-dependent behaviour. Fig. 4e reveals the PL emission spectra of the CDs solid dispersed in water, DMF, acetone, ethanol and toluene. These results are consistent with the fluorescent patterns in Fig. 4b. We also investigated the relationship between polarity values and PL intensity (Fig. S4, ESI†). With the polarity values varying from 2.4 (toluene) to 10.2 (water), the PL intensity increases from 50.47 a.u. to 860 a.u. The abundant polar groups such as hydroxyl and carbonyl on the surfaces of the CDs can explain this phenomenon. These functional polar groups endow the CDs with different solubility in diverse solvents, which might account for the different PL intensity.36
Temperature sensitivity of PNIPAM/CDs hybrids
To further investigate the capability of CDs, we synthesized PNIPAM/CDs hybrids through the crosslinking of NIPAM and CDs, and the composites were used as a temperature switch. PNIPAM is a typical thermo-sensitive polymer, which can exhibit a coil-globule transition in aqueous solution at a lower critical solution temperature (LCST) at around 32 °C.44 Fig. S5a (ESI†) demonstrates the PL spectra of the PNIPAM hybrid and PNIPAM/CDs hybrids under 360 nm, which shows obvious difference. The PL intensity of PNIPAM/CDs hybrids as high as 490 a.u., the PL intensity of the PNIPAM hybrid nearly close to zero, and further imply that the PNIPAM hybrid itself fluorescence can be excluded. Fig. 5a shows the PL spectra of the PNIPAM/CDs hybrids at various temperature from 20 to 40 °C. The PL intensity of the hybrids decreased with temperature increase, and a sharp decline can be found at around 32 °C. It should be noted that the temperature-dependent PL intensity behaviour is reversible during 6 heating–cooling cycles, facilitating its further use in practice (Fig. 5b). Moreover, we explained the mechanism of this phenomenon. As outlined in Fig. 5c, when cooled (off), the hydrogel network was homogeneous, and the CDs were uniformly dispersed throughout it. However, when heated (on), intrachain collapse occurred, and the PNIPAM/CDs hydrogel underwent a sharp phase transition, thus the gel network became heterogeneous and opacity (Fig. S5b, ESI†), leading to the aggregation of CDs along with decrease in PL intensity.44,45 The PL intensity switched under variable temperature, making it possible to develop CDs-based thermo-sensitive devices or sensors.Fluorescent probes for sensitive detection of Fe3+
Ions play an important role in biological system, especially Fe3+. It not only provides oxygen-carrying capacity to heme, but acts as a cofactor in many enzymatic reactions involved in the mitochondrial respiratory chain.46 To extend the application of nitrogen-doped CDs, a sensing system for metal ions was investigated. The PL intensity in the presence of representative metal ions was investigated under the same conditions, including Fe2+, Ca2+, Cd2+, Cr3+, Co2+, Na+, Cu2+, Fe3+, Pb2+, Zn2+, Al3+, Mg2+, Ba2+ and Hg2+, as shown in Fig. S6a (ESI†). From the fluorescence intensity histogram (Fig. S6b, ESI†), slight fluorescence quenching can be observed for Fe2+, Ca2+, Cd2+, Cr3+, Co2+, Na+, Cu2+, Pb2+, Zn2+, Al3+, Mg2+, Ba2+ and Hg2+. This phenomenon can be attributed to nonspecific recognition function between the carboxylic groups on the surface of the CDs and the metal ions.22 Significant fluorescence quenching was observed for Fe3+. The high affinity of N and O on the surface of the nitrogen-doped CDs for complex formation with Fe3+ can account for the fluorescence quenching effect.47To further investigate the sensitivity of this sensing system, the PL intensity of nitrogen-doped CDs with various concentrations of Fe3+ were investigated. Fig. 6a shows the PL intensity of CDs gradually decrease with the increasing of Fe3+ concentration, revealing that the sensing system can efficiently quench the PL of the nitrogen-doped CDs. Fig. 6b reveals the dependence of F0/F on the concentrations of Fe3+ ions in the range from 0 to 300 μM, where F0 and F are the fluorescence intensity in the absence and with different concentrations of Fe3+ ions, respectively. More importantly, the quenching efficiency follows the Stern–Volmer equation.48 The obtained Stern–Volmer plot fits a linear equation at concentrations over the range from 0–300 μM with a good linear correlation (R2 = 0.9925). The detection limit (calculated according to a signal-to-noise ratio of S/N = 3) was estimated to be 2.5 μM.
 | ||
| Fig. 6 (a) PL emission spectra of CDs in the presence of Fe3+ ions at different concentrations. (b) The linear relationship between F0/F and Fe3+ concentration. | ||
Conclusions
In summary, nitrogen-doped CDs were successfully synthesized through a hydrothermal process by using N-riched PAMAM dendrimer as the precursor. The morphology, composition and fluorescence properties of the CDs were thoroughly investigated. The CDs depict excellent monodispersity and solubility in water with the QY of 40%. On the other hand, the CDs demonstrate sensitivity detection of Fe3+ ions, and the detection limit can reach to 10−6 M (ppm level). To be more practical applications, the as-prepared CDs could be the ink candidates of nano-printing patterns, which are of high fluorescence. What's more, we have found the pH values and solvents used in the ink have great influence on the intensity of their fluorescence. More interestingly, we prepared PNIPAM/CDs hybrids, which exhibit temperature switch property. Its reversibly sensitive external temperatures range from 20 °C to 40 °C, making it possible to develop CDs-based thermo-sensitive devices or sensors. This strategy might provide an available pathway to handy access of nitrogen-doped CDs.Acknowledgements
This work was supported by National Natural Science Foundation of China (21474052), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- S. T. Yang, L. Cao, P. G. Luo, F. Lu, X. Wang, H. Wang, M. J. Meziani, Y. Liu, G. Qi and Y. P. Sun, J. Am. Chem. Soc., 2009, 131, 11308–11309 CrossRef CAS PubMed.
- J. Ge, Q. Jia, W. Liu, L. Guo, Q. Liu, M. Lan, H. Zhang, X. Meng and P. Wang, Adv. Mater., 2015, 27, 4169–4177 CrossRef CAS PubMed.
- M. Zheng, S. Liu, J. Li, D. Qu, H. Zhao, X. Guan, X. Hu, Z. Xie, X. Jing and Z. Sun, Adv. Mater., 2014, 26, 3554–3560 CrossRef CAS PubMed.
- S. Karthik, B. Saha, S. K. Ghosh and N. D. Pradeep Singh, Chem. Commun., 2013, 49, 10471–10473 RSC.
- X. Guo, C. F. Wang, Z. Y. Yu, L. Chen and S. Chen, Chem. Commun., 2012, 48, 2692–2694 RSC.
- L.-H. Mao, W.-Q. Tang, Z.-Y. Deng, S.-S. Liu, C.-F. Wang and S. Chen, Ind. Eng. Chem. Res., 2014, 53, 6417–6425 CrossRef CAS.
- Q.-L. Chen, C.-F. Wang and S. Chen, J. Mater. Sci., 2012, 48, 2352–2357 CrossRef.
- H. Li, X. He, Z. Kang, H. Huang, Y. Liu, J. Liu, S. Lian, C. H. Tsang, X. Yang and S. T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430–4434 CrossRef CAS PubMed.
- J. Briscoe, A. Marinovic, M. Sevilla, S. Dunn and M. Titirici, Angew. Chem., Int. Ed., 2015, 54, 4463–4468 CrossRef CAS PubMed.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2015, 44, 362–381 RSC.
- J. Wang, C. F. Wang and S. Chen, Angew. Chem., Int. Ed., 2012, 51, 9297–9301 CrossRef CAS PubMed.
- S. L. Hu, K. Y. Niu, J. Sun, J. Yang, N. Q. Zhao and X. W. Du, J. Mater. Chem., 2009, 19, 484–488 RSC.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang and H. Wang, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- H. Ming, Z. Ma, Y. Liu, K. Pan, H. Yu, F. Wang and Z. Kang, Dalton Trans., 2012, 41, 9526–9531 RSC.
- H. Ding, L.-W. Cheng, Y.-Y. Ma, J.-L. Kong and H.-M. Xiong, New J. Chem., 2013, 37, 2515 RSC.
- W. Shi, Q. Wang, Y. Long, Z. Cheng, S. Chen, H. Zheng and Y. Huang, Chem. Commun., 2011, 47, 6695–6697 RSC.
- S. Hu, R. Tian, L. Wu, Q. Zhao, J. Yang, J. Liu and S. Cao, Chem.–Asian J., 2013, 8, 1035–1041 CrossRef CAS PubMed.
- R. Ye, C. Xiang, J. Lin, Z. Peng, K. Huang, Z. Yan, N. P. Cook, E. L. Samuel, C. C. Hwang, G. Ruan, G. Ceriotti, A. R. Raji, A. A. Marti and J. M. Tour, Nat. Commun., 2013, 4, 2943 Search PubMed.
- S. Yang, J. Sun, P. He, X. Deng, Z. Wang, C. Hu, G. Ding and X. Xie, Chem. Mater., 2015, 27, 2004–2011 CrossRef CAS.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 52, 3953–3957 CrossRef CAS PubMed.
- X. Gao, Y. Lu, R. Zhang, S. He, J. Ju, M. Liu, L. Li and W. Chen, J. Mater. Chem. C, 2015, 3, 2302–2309 RSC.
- D. Qu, Z. Sun, M. Zheng, J. Li, Y. Zhang, G. Zhang, H. Zhao, X. Liu and Z. Xie, Adv. Opt. Mater., 2015, 3, 360–367 CrossRef CAS.
- Y. Li, Y. Zhao, H. Cheng, Y. Hu, G. Shi, L. Dai and L. Qu, J. Am. Chem. Soc., 2012, 134, 15–18 CrossRef CAS PubMed.
- M. K. Barman, B. Jana, S. Bhattacharyya and A. Patra, J. Phys. Chem. C, 2014, 118, 20034–20041 CrossRef CAS.
- W. Wang, Y. C. Lu, H. Huang, A. J. Wang, J. R. Chen and J. J. Feng, Biosens. Bioelectron., 2015, 64, 517–522 CrossRef CAS PubMed.
- Q. Xu, P. Pu, J. Zhao, C. Dong, C. Gao, Y. Chen, J. Chen, Y. Liu and H. Zhou, J. Mater. Chem. A, 2015, 3, 542–546 RSC.
- H. Li, F.-Q. Shao, S.-Y. Zou, Q.-J. Yang, H. Huang, J.-J. Feng and A.-J. Wang, Microchim. Acta, 2015, 183, 821–826 CrossRef.
- W. Wang, Y. C. Lu, H. Huang, J. J. Feng, J. R. Chen and A. J. Wang, Analyst, 2014, 139, 1692–1696 RSC.
- Y. Du and S. Guo, Nanoscale, 2016, 8, 2532–2543 RSC.
- X. Chen, Q. Jin, L. Wu, C. Tung and X. Tang, Angew. Chem., Int. Ed., 2014, 53, 12542–12547 CAS.
- J. Zhang, Y. Yuan, G. Liang and S.-H. Yu, Adv. Sci., 2015, 2, 1500002–1500007 CrossRef PubMed.
- Y. Ke, B. Garg and Y.-C. Ling, RSC Adv., 2014, 4, 58329–58336 RSC.
- L. Li, G. Wu, G. Yang, J. Peng, J. Zhao and J. J. Zhu, Nanoscale, 2013, 5, 4015–4039 RSC.
- S.-S. Liu, C.-F. Wang, C.-X. Li, J. Wang, L.-H. Mao and S. Chen, J. Mater. Chem. C, 2014, 2, 6477 RSC.
- D. Qu, M. Zheng, L. Zhang, H. Zhao, Z. Xie, X. Jing, R. E. Haddad, H. Fan and Z. Sun, Sci. Rep., 2014, 4, 5294 CrossRef CAS PubMed.
- H. Ding, S. B. Yu, J. S. Wei and H. M. Xiong, ACS Nano, 2016, 10, 484–491 CrossRef CAS PubMed.
- Z. Yang, M. Xu, Y. Liu, F. He, F. Gao, Y. Su, H. Wei and Y. Zhang, Nanoscale, 2014, 6, 1890–1895 RSC.
- G. Eda, Y. Y. Lin, C. Mattevi, H. Yamaguchi, H. A. Chen, I. S. Chen, C. W. Chen and M. Chhowalla, Adv. Mater., 2010, 22, 505–509 CrossRef CAS PubMed.
- L. Zhu, Y. Yin, C.-F. Wang and S. Chen, J. Mater. Chem. C, 2013, 1, 4925 RSC.
- F. Yuan, L. Ding, Y. Li, X. Li, L. Fan, S. Zhou, D. Fang and S. Yang, Nanoscale, 2015, 7, 11727–11733 RSC.
- C. Zheng, X. An and J. Gong, RSC Adv., 2015, 5, 32319–32322 RSC.
- J. Li, X. Hong, Y. Liu, D. Li, Y. W. Wang, J. H. Li, Y. B. Bai and T. J. Li, Adv. Mater., 2005, 17, 163–166 CrossRef CAS.
- L. Zhou, B. He and J. Huang, Chem. Commun., 2013, 49, 8078–8080 RSC.
- J. Liu, Y.-Q. Xie, Q. Lin, B.-B. Shi, P. Zhang, Y.-M. Zhang and T.-B. Wei, Sens. Actuators, B, 2013, 186, 657–665 CrossRef CAS.
- J. Yu, C. Xu, Z. Tian, Y. Lin and Z. Shi, New J. Chem., 2016, 40, 2083–2088 RSC.
- Y. Song, S. Zhu, S. Xiang, X. Zhao, J. Zhang, H. Zhang, Y. Fu and B. Yang, Nanoscale, 2014, 6, 4676–4682 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12261b |
| This journal is © The Royal Society of Chemistry 2016 |



