Large scale applications of immobilized enzymes call for sustainable and inexpensive solutions: rice husks as renewable alternatives to fossil-based organic resins†
L. Corici‡
a,
V. Ferrariob,
A. Pellis§
b,
C. Ebertb,
S. Lotteriab,
S. Cantone¶
a,
D. Voinovichb and
L. Gardossi*b
aSPRIN S.p.a., Via Flavia 23/1, 34148, Trieste, Italy
bLaboratory of Applied and Computational Biocatalysis, Dipartimento di Scienze Chimiche e Farmaceutiche, Università degli Studi di Trieste, Piazzale Europa 1, 34127, Trieste, Italy. E-mail: gardossi@units.it
First published on 23rd June 2016
Abstract
Despite the extensive efforts of the scientific community towards the development of a vast variety of immobilization methods, there is a limited number of immobilized biocatalysts used at an industrial scale. Most often, cost issues prevent the transfer of methodologies to large scale but more recently sustainability criteria are also becoming increasingly relevant, so that petroleum based carriers for enzyme immobilization appear unsuitable for responding to the new challenges of green and renewable chemistry. Here we report, for the first time, a preliminary overview of the potential of rice husks as carriers to be employed for both physical and covalent immobilization of enzymes. The data indicate that the chemical versatility of this lignocellulosic biomass, containing also silica, opens wide scenarios for optimizing different immobilization procedures requiring minimal pre-treatments and applicable to various enzymes and process conditions. The mechanical and chemical robustness of rice husks, along with their virtually unlimited availability worldwide, make this inexpensive natural matrix a promising candidate for replacing organic fossil-based carriers for enzyme immobilization.
Introduction
Immobilized biocatalysts have the potential of enabling continuous processing, lowering production costs and waste stream generation. Nevertheless, immobilized enzymes represent only a minor fraction of the overall enzyme market and relatively few processes employing immobilized enzymes have been successfully commercialized. These issues were extensively discussed by DiCosimo et al., who explained how, from an industrial perspective, the cost of most enzymes is often only a minor component in overall process economics.1 Consequently, the cost of producing an immobilized biocatalyst preparation must be justified by significant returns besides the fact that immobilized enzymes can be reused.Beyond the economic issues, immobilization is mandatory for some enzyme applications as, for instance, in processes in organic phases where hydrophilic native proteins are prone to aggregate when hydrated or solvated by polar components. Prevention of protein contamination is one further motivation that induces pharma and cosmetic sectors to employ immobilized biocatalysts.2
Indeed, the number of immobilized enzymes used on scale larger than 10 kton per year is limited and includes mostly hydrolases, although the most relevant case is represented by immobilized glucose isomerase that enables the production of high fructose corn syrup on a 107 ton scale per year.1
Despite the evidence that the implementation of processes employing immobilized enzymes has not met – till now – the initial optimistic expectations, recent analysis indicate that there is an enormous catalytic potential waiting to be exploited for the production of renewables chemicals and fuels from biomass resources under mild and sustainable conditions. Such challenge must be addressed keeping in mind that commodity chemicals typically have very low added value, so that cheap carriers and protocols, together with high reusability are the factors that will determine the success of immobilized enzymes in biobased commodity chemistry.3 Furthermore, in the perspective of achieving fully renewable and sustainable bio-based products, environmental factors are gaining increasing relevance in determining the overall viability of the biocatalytic process, as demonstrated by several recent applications of Life Cycle Analysis (LCA) methodology to the study of the impact of enzyme immobilization and carriers in particular. For example, it has been pointed out that the production of epoxy activated methacrylic resins represents the primary greenhouse gas emission source for the immobilized enzymes (51–83%), primarily because of the fossil based raw materials, namely glycidyl methacrylate and ethylene dimethyl acrylate.4
These factors indicate that there is a need of a more holistic analysis of factors that will drive the next generation of immobilized biocatalysts, since costs and sustainability issues are rarely discussed in the abundant scientific literature of the last decades covering immobilization methods.
Nowadays, an array of commercially available acrylic and styrenic resins provides the most straightforward route for immobilizing proteins on insoluble carriers, since their physical and chemical properties have been optimized by the chemical industry by transferring well-established knowhow of chromatographic sector. However, as also reported above,4 the polymer industry is under pressure to mitigate the environmental cost of petrol-based plastics5 and the UN Environmental Programme estimated that the overall natural capital cost of plastic use in the consumer goods sector each year is US $ 75 billion, with 30% of the natural capital costs due to greenhouse gas emissions from raw material extraction and processing (http://www.unep.org/yearbook/2014/).
Natural biopolymers from agricultural and food chain biomass may represent attractive alternatives responding to the pressing challenges of a circular economy, as long as they do not suffer from biological safety aspects, they are largely available on a constant basis at low prices and they do not require complex pre-treatments. On a global scale, the two highest-volume agricultural residues are rice husk (RH) and sugarcane bagasse.6 Rice is a typical crop not only in Asia but also in Africa, Central and South America and some European regions. For every 4 t of rice harvested, 1 t of husk is produced, amounting to 120 Mt of rice husk per year. Of this, only 20 Mt are currently used, leaving around 100 Mt accessible to be converted into fuels or chemicals at large scale and with continuous supply.
In the present study we have explored the potential of milled RH as carrier for the immobilization of three different enzymes. The agricultural residues were employed after minimal pre-treatments and maintaining the whole composite matrix made, besides SiO2, by three different biopolymers, namely cellulose, hemicellulose and lignin. Different functionalizations of the cellulose components were tested as routes for covalent anchoring of proteins. The non-optimized RH formulations of adsorbed and covalently immobilized Candida antarctica lipase B (CaLB) were also tested in solvent free esterification and polycondensation reactions, demonstrating competing properties when compared with commercial styrenic and methacrylic preparations. The promising results motivate further investigation of this agricultural residue towards the optimization of protocols for immobilization as well functionalization of RH matrix.
Results and discussion
Rice husk (RH) was taken into consideration with its complex chemical matrix that offers multiple opportunities for developing immobilization strategies. Lignin provides an aromatic component that is expected to promote hydrophobic interactions. The cellulose fraction provides a structural platform for chemical modifications and functionalizations suitable for covalent anchoring of proteins. Finally, silica confers remarkable mechanical strength to the composite material. Previous studies7 indicated that RH is composed for more than 20% by SiO2 and about 5% of different inorganic oxides (Al2O3, Fe2O3, CaO, MgO and MnO2), whereas 70–80% is represented by organic matter and moisture. The siliceous component of RH has been already exploited in the preparation of inorganic catalysts8 as well as in the formulation of biocatalysts.9 Wong and co-workers reported that rice husk contains around 10–20% of silicon dioxide depending on the soil in which the stalks grow.10 Rice can deposit amorphous silica on cell walls, forming silica-cuticle and silica-cellulose double layers on the surface of leaves, stems and husks.11 In rice husks, aggregations of small and large silica particles are formed on the intercellular layers and surface of cell walls.The hydroxyl groups of silica in the RH can be functionalized with different silylating agents, for example with 3-(aminopropyl)triethoxysilane.12 The silica fraction has been also exploited for the synthesis of titanium silicon oxides by utilizing rice husk with calcination method to combine natural hierarchical porous structure with applications in photocatalysis.8
In this first explorative study, we intended to investigate the potential of RH as a whole, to minimize the economic impact of processing. Initially, we focused the attention on the possibility of immobilizing enzymes via adsorption on RH particles. Afterwards, we considered different methods for covalent immobilization either via cross-linking or by introducing proper functional groups on the RH carrier.
Characterization of rice husk
The investigation started by determining the composition of the organic fraction of RH. The first step consisted of grinding and sieving (see ESI, Fig. S1†) to obtain particle sizes between 150 and 300 μm, comparable to the dimension of commercial beads generally employed as carriers. Soxhlet extraction (see Materials and methods section) was used to remove wood extractives such as fatty acids, sterols, waxes, sugars, coloring matter, which could interfere with the intended technological applications of the material. In order to understand actual advantages coming from the use of RH, similar treatments and analytical procedures were also applied to nutshells (NS), a lignocellulosic biomass of comparable composition13 and a comparison of the properties of the two raw materials are summarized in Table S1 of ESI.†In agreement with previous studies,14 extractives recovered from RH were below 1.8% w w−1, and the absence of coloured fractions was confirmed by the observation that the colour of RH does not change significantly (see ESI, Fig. S2a and b†). The chemical composition of organic fraction was determined according to the literature15 and it is summarized in Table 1. Generally, the chemical composition of rice husk may change depending on the type of paddy, climatic or geographical conditions16 and such factors determine differences in terms of cellulose and lignin composition observed when compared with other investigations.15 In the case of NS, similar results were obtained in terms of lignin (32.4%) and hemicellulose (21.3%), but a lower content of cellulose (29.3%) was found (ESI, Table S1†). Most importantly, we observed, in agreement with previous reports,13 that more than 10% of organic matter is removed during extractive procedure (ESI, Table S1†) and that NS fibres changed from brown to lighter brown color after extractive treatments (see ESI, Fig. S2c and d†).
| Rice husk composition | % wt |
|---|---|
| Moisture | 8.2 |
| Cellulose | 46.5 |
| Lignin | 31.9 |
| Pentosans | 22.1 |
A preliminary spectroscopic characterization of RH and NS was carried out using FT-IR spectroscopy (see ESI, paragraph S3†). The spectra for both biomasses, present the typical adsorption bands of cellulose, hemicellulose and lignin. However, the absorbance associated with the typical values of cellulose (3500–3000 cm−1, 1730–1710 cm−1, 1270, 1156, 1075 and 896 cm−1) are more evident in the spectrum of RH and this observation confirms that RH contains a higher percentage of cellulose as compared to NS, making RH more suitable for our functionalization and immobilization purposes.
The morphological structure of both natural matrices has been investigated by scanning electron microscopy analysis that shows the fibrous nature with a smooth surface of RH (Fig. 1a and b). After milling (Fig. 1c and d), the fibers become irregular and the outer surface is highly roughened. Moreover, the external epidermal cells are arranged in linear ridges, which are punctuated with prominent domes where the silica is predominantly localized, whereas a lower amount of silica can be found in other regions of the rice husk. Therefore, the high content of silica on outer epidermis provides strength and stiffness to the material.
 | ||
| Fig. 1 Scanning electron micrographs of (a and b) rice husk fibres at different orders of magnification, (c and d) milled rice husk fibres. | ||
The scanning electron micrographs of NS showed more regular size and shape of the particles if compared to rice husk fibers, but with a similarly irregular surface (see ESI, Fig. S4†).
Functionalization of RH and immobilization of enzymes according to different methods
In order to understand the potential of this new complex renewable matrix, three different enzymes were selected for immobilization: lipase B from Candida antarctica (CaLB), thermolysin protease from Geobacillus sp. (tpG) and the invertase from Saccharomyces cerevisiae (ISc).17 The enzymes were selected in view of their wide use at industrial scale.18 CaLB (3.1.1.3) is widely employed in the production of fine chemicals for its selectivity but also in the production of esters for body care sector. Overall, lipases have a wide industrial impact in the food, pharma and cosmetic sectors but their catalytic potential is far from being fully exploited for the production of commodities or biodiesel.19–21Invertase or β-D-fructofuranosidase (E.C. 3.2.1.26) from Saccharomyces cerevisiae catalyzes sucrose hydrolysis. This reaction results in an equimolar mixture of β-D-glucose and β-D-fructose, known as invert syrup, which is widely used in food and beverage industries. Immobilized invertase is used for continuous hydrolysis of sucrose as the resulting shifts in the pH can be used to prevent the formation of oligosaccharides by the transferase activity associated with the soluble enzyme.22 This enzyme is also used for the manufacture of plasticizing agents used in cosmetics, pharmaceutical and paper industries as well as enzyme electrodes for the detection of sucrose. Invertase has been selected because there are studies in the literature describing its immobilization on pre-treated rice husk. Thermolysin from Geobacillus sp. (tpG, E.C.3.4.24.27) is a zinc protease massively used in detergents. It is also employed, in immobilized form, for the industrial production of Aspartame and for the hydrolysis of milk proteins to obtain bioactive peptides.1,23
The structural models reported in Fig. 2 highlight the lysine residues that are generally the major responsible for the anchorage on solid carrier through covalent immobilization.24 The three enzymes, because of their natural substrates and functions, have quite different superficial features. As most globular proteins, ISc and tpG display a prevalently hydrophilic surface exposed to the aqueous environment and hydrophilic substrates. On the contrary, it is known that lipases are characterized by unusually large hydrophobic surfaces surrounding the active site entrance which are consistent with the necessity of approaching the lipidic natural substrates.25
Indeed, a consistent improvement was observed by carrying out the immobilization in rapeseed oil as previously documented.20 The hydrophobic medium promotes the partition of the protein on the RH that is less hydrophobic than the rapeseed oil, thus favouring its adsorption. The amount of protein loaded on RH was set on the basis of the capacity of the matrix of adsorbing the aqueous solution added during the immobilization procedure (see Materials and methods section). The presence of residual oil on the biocatalyst did not allow the quantitative determination of the protein loaded on RH. It is important to underline that the preparations formulated through simple adsorption were not tested in terms of hydrolytic activity since we have previously demonstrated how part of the protein detaches from the support during the assay in aqueous medium.20 Nevertheless, the preliminary results indicate that only this methodology has some potential utility for physical immobilization of CaLB on RH.
On the contrary, attempts directed to the improvement of the adsorption of CaLB on RH in aqueous media were unsuccessful. More specifically, the pre-treatment of the carrier with polyethylene imine (PEI)27 led to less than 10% of protein loading (Table 2, entry 3). In this case, after adsorption on RH, CaLB was crosslinked with glutaraldehyde to avoid protein leaching and finally the hydrolytic and synthetic activities were tested. The fraction of adsorbed enzyme retained (after crosslinking) was less than 10% of the original activity.
| Entry | Carrier | Method of immobilization | Loadinga (U gdry−1) | Protein loaded (%) | Synthetic activityb (U gdry−1) |
|---|---|---|---|---|---|
| a Tributyrin units (TBU) calculated with the hydrolytic assay reported in the experimental section.b Synthetic activity determined in the synthesis of propyl laurate.c Not determined because of the interference of rapeseed oil with the assay. | |||||
| 1 | RH | Adsorbed in buffer | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
<5 | <5 |
| 2 | RH | Adsorbed in rape seed oil20 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
n.d.c | 1008 |
| 3 | RH pretreated with PEI | Adsorbed and crosslinked27 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8 | 470 |
| 4 | PS/DVB | Adsorbed | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
65 | 7500 |
| 5 | MA/DVB | Adsorbed | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
72 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 6 | PS/DVB | Adsorbed and crosslinked | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
65 | 3480 |
As a term of reference, we adsorbed CaLB on a commercial polystyrene/divinyl benzene resin (PS/DVB) Diaion HP20-L and also on a methacrylic-divinyl benzene (MA/DVB) resin (Lewatit) using an optimized protocol (see Materials and methods section). In these cases the synthetic activities were between 7500 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U gdry−1 respectively, but starting from a higher enzyme loading (50
000 U gdry−1 respectively, but starting from a higher enzyme loading (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 TBU). It must be noted that also in these cases the performance of the adsorbed CaLB preparations were not expressed in terms of recovered hydrolytic activity because the adsorbed enzyme would have leached off the carrier during the hydrolytic assay.20
000 TBU). It must be noted that also in these cases the performance of the adsorbed CaLB preparations were not expressed in terms of recovered hydrolytic activity because the adsorbed enzyme would have leached off the carrier during the hydrolytic assay.20
Upon crosslinking, CaLB on HP20-L loses more than half of its synthetic activity (Table 2, entry 6). In conclusion, RH demonstrated a low affinity towards CaLB when used in aqueous medium, most probably because of its lower hydrophobic nature as compared to PS and MA/DVB. However, by carrying out the immobilization in rapeseed oil the adsorption of the protein on the matrix is improved and this opens the perspectives for the use of this renewable carrier in hydrophobic media. More specifically, this method would allow the in situ immobilization of CaLB on RH for applications in hydrophobic media or oils, thus reducing complicated and costly steps for the formulation of the biocatalyst. The protocol employing vegetable oil as immobilization medium implies that no enzyme is lost during the procedure because no partition occurs between an aqueous and a solid phase but rather the dissolved protein is adsorbed onto the carrier.21 However, the loading capacity is limited by the capacity of RH to adsorb water (about 42%, see Materials and methods section) but the inexpensive carrier and procedure make possible to employ larger amounts of biocatalysts endowed with lower specific activity but distributed on a wider surface. This result meets the main objective of the present study, namely the identification of renewable, inexpensive carriers, which should also largely available for applications in the production of bio-based commodities. Products such as biodiesel, transesterified oils, emollients and oligo/polyesters are preferentially produced in solvent-less bulky system. We have previously demonstrated that such processes often suffer from mass transfer limitations and the use of immobilized enzymes characterized by high protein loadings does not overcome the problem, while increases the economic impact of the biocatalyst. In such cases, the reaction efficiency is mainly determined by the extension of the accessible surface of the biocatalyst. Widening the porosity of carriers would be of scarce help, since large and viscous compounds partition into the pores with difficulty. Instead, the goal is achievable by employing a larger biocatalyst volume in the reaction mixture, which turns to be beneficial for mass transfer and reaction kinetics.19
Differently from CaLB, tpG and ISc showed a higher affinity towards RH when adsorbed starting from aqueous medium (Table 3), in agreement with their predominant hydrophilic surface.
| Entry | Enzyme | Method of immobilization | Loading (U gdry−1) | Protein loaded (%) | Hydrolytic activity (U gdry−1) |
|---|---|---|---|---|---|
| a Hydrolysis of casein.b Hydrolysis of sucrose.c Adsorbed in buffer with 0.75 M NaCl. | |||||
| 7 | tpG | Adsorbed and crosslinked | 840 | 30 | 5.6a |
| 8 | tpG | Adsorbed and crosslinkedc | 750 | 25 | 8.2a |
| 9 | ISc | Adsorbed and crosslinked after pretreatment with PEI | 2000 | 79b | 58b |
In the case of tpG the enzyme was crosslinked after adsorption, in analogy with the classical method reported by Nakanishi et al.,27 which used the enzyme adsorbed on Amberlite XAD-7 (acrylic anionic exchange resin) and crosslinked for the production of aspartame. The work was focused mainly on enzyme stability whereas the recovered activity was not discussed. The performance of immobilized tpG was evaluated in the hydrolysis of casein. Similar results were obtained when the protease was immobilized with a higher ionic strength (0.75 M NaCl), which has been reported to improve the enzyme solubility and prevent aggregates (Table 3, entry 8).28 On the light of the modest commercial cost of tpG and RH, the low percentage of recovered activity, might be acceptable only if accompanied by stability under operational conditions.
As a term of comparison, we have carried out the covalent immobilization of tpG also on a methacrylic commercial resin (see ESI†) and comparable results were obtained, which suggest the unsuitability of this enzyme for being covalently immobilized. Nevertheless, it must be noted that the economic impact of commercial organic carriers is above 100 euro per kg of immobilized enzyme, whereas RH is available in tons at a price close to zero.
The immobilization of ISc was carried out according to the procedure reported by D'Souza et al., who pretreated the RH with PEI (Table 3, entry 9) to promote electrostatic interactions between proteins and the carrier before crosslinking.3 Despite the 79% of protein loaded on the carrier, the fraction of recovered activity (about 5% determined in the hydrolysis of a sucrose solution) was much lower when compared to data reported by D'Souza work (21–56%). It must be noted that the previous study did not report information on the size of RH particles nor on their compositions, so that a direct comparison of the two set of data is not feasible.
The immobilization of enzymes through covalent binding on functionalized rice husk was guided by the observation that cellulose is the rice husk main component and it can be reacted with various techniques, rendering the activated derivatives useful for enzyme immobilization purposes. The first functionalization procedure consisted of cellulose oxidation by sodium periodate.29 The reaction is highly specific, cleaving the C2–C3 bond of glucose molecules and converting the hydroxyl groups into aldehyde groups. Enzyme molecules were then covalently linked to aldehyde groups via the lysine (Lys) residues (Fig. 3a) and formation of imine bonds. Secondly, the oxidized RH was also reacted with a diamino spacer, and the latter was then activated with glutaraldehyde (Fig. 3b). The enzyme was finally anchored on the aldehyde groups via the Lys residues.
In the case of ISc, the immobilized preparation obtained by covalent binding of enzyme to aldehyde activated support and diamino spacer hexamethylene diamine (HMDA) showed the best performances, with an activity of 275 U gdry−1 and 68% of the protein bounded (Table 4, entry 11).
| Entry | Functionalization | Immobilization method | Loading (U gdry−1) | Hydrolytic activity (U gdry−1) | Protein loaded (%) |
|---|---|---|---|---|---|
| 10 | Oxidized RH | Covalent via imine bond | 2000 | 9.2 | 25 |
| 11 | Oxidized RH + HMDA spacer activated with glutaraldehyde | Covalent via imine bond | 2000 | 275 | 68 |
The same method led to good results for the covalent immobilization of CaLB. It is noteworthy that the introduction of a long spacer improves the performance of the enzyme both in hydrolytic and synthetic reactions, most probably by avoiding steric hindrance. Indeed, CaLB displays half of the hydrolytic activity when it is anchored directly on the oxidized cellulose. The HMDA spacer causes a threefold increase of the loading of ISc and, more significantly, about 50-fold higher activity. The importance of introducing a sufficiently long spacer on RH surface is supported also by the observation that the activity of CaLB drops significantly (Table 5, entry 14) with the use of a shorter spacer, ethylene diamine (EDA), despite a 58% of protein loaded on RH. Apparently, it is not sufficient to increase the amount of loaded enzyme on RH but the spacer must prevent the crowding of the molecules on the surface, thus enabling the access of the substrate to the active sites.
| Entry | Functionalization | Method of immobilization | Loading (U gdry−1) | Protein loaded (%) | Hydrolytic activity (U gdry−1) | Synthetic activity (U gdry−1) |
|---|---|---|---|---|---|---|
| a HMDA: 0.18 mol gdry−1.b EDA: 0.18 mol gdry−1.c HMDA: 0.60 mol gdry−1. | ||||||
| 12 | Oxidized RH30 | Imine bond | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
n.d. | 120 | 1057 |
| 13 | Oxidized RH + HMDA spacer activated with glutaraldehyde31 | Imine bonda | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
49 | 243 | 2178 |
| 14 | Oxidized RH + EDA spacer activated with glutaraldehyde | Imine bondb | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
58 | 148 | 1349 |
| 15 | Oxidized RH + HMDA spacer activated with glutaraldehyde | Imine bondc | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
34 | 273 | 1183 |
| 16 | Oxidized RH + HMDA spacer activated with glutaraldehyde | Imine bondc | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
35 | 317 | 1288 |
| 17 | Epoxy activated RH using GPTMS32 | Amine attack on epoxide | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
70 | 41 | 550 |
| 18 | RH preactivated with ATES + glutaraldehyde9 | Imine bond | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
19 | 149 | 892 |
| 19 | EC-EP/S33 | Amine attack on epoxide | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>90 | 2400 | 2000 |
Overall, the data indicate that using 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 TBU units per g of dry RH, the carrier is already oversaturated because half of the protein loading (Table 5, entry 16) leads to comparable activity. Consequently, the use of higher concentrations of diamino spacer (Table 5, entry 15) does not lead to any benefit. Preliminary data indicate that using only 10
000 TBU units per g of dry RH, the carrier is already oversaturated because half of the protein loading (Table 5, entry 16) leads to comparable activity. Consequently, the use of higher concentrations of diamino spacer (Table 5, entry 15) does not lead to any benefit. Preliminary data indicate that using only 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units of CaLB but prolonging the immobilization time to 48 h more than 70% of protein get fully covalently attached to RH while an hydrolytic activity of 310 U gdry−1 is retained. Evidence of the covalent immobilization is available in ESI (Fig. S10†). These observations indicate that further optimization studies should pursue the direction of preparing CaLB formulations with lower loadings, with the consequent economic advantages.19
000 units of CaLB but prolonging the immobilization time to 48 h more than 70% of protein get fully covalently attached to RH while an hydrolytic activity of 310 U gdry−1 is retained. Evidence of the covalent immobilization is available in ESI (Fig. S10†). These observations indicate that further optimization studies should pursue the direction of preparing CaLB formulations with lower loadings, with the consequent economic advantages.19
The results were compared with the activities, both hydrolytic and synthetic, measured for a formulation of CaLB immobilized on a commercial epoxy-activated methacrylic resin according an optimized protocol (Table 5, entry 19). It is evident that CaLB displays similar synthetic activity when immobilized on RH or EC-EP/S whereas the hydrolytic activity is considerably lower on RH. This behavior might be ascribed to partition or diffusion phenomena, or the different porosity of the carriers. On that respect, we evaluated the water adsorption capacity of RH particles according to the protocol reported in the experimental Materials section and resulted to be 42.6%, as compared to the 63% of EC-EP/S resin. The finding that RH, despite containing cellulose and silica, has lower water capacity when compared to methacrylic (macroporous) beads suggest the presence of less structures pores in the biomaterial. Furthermore, wettability studies usually involve the measurement of contact angles as the primary data, which indicates the degree of wetting when a solid and liquid interact. Small contact angles (<90°) correspond to high wettability, while large contact angles (>90°) correspond to low wettability.30
The mean contact angle for RH (n = 3) was 58.10 ± 11.59, indicating a powder prone to aqueous wetting. Duplicates of CaLB immobilized on oxidized RH through the HMDA spacer (entry 13), here named RH-CaLB showed a reproducible synthetic activity of about 2000 U gdry−1. The hydrolytic assay, carried out in aqueous buffer, indicated that the preparation retains about 90% of its activity upon 10 recycles, thus confirming that the CaLB is effectively covalently linked to the matrix (Fig. 4).
 | ||
| Fig. 4 Activity retained by the RH-CaLB preparation (entry 13) upon 10 recycles in the standard tributyrin hydrolysis assay. | ||
As alternative functionalization strategies, the cellulose fraction of RH was also treated with glicidoxypropyltrimethoxysilane (GPTMOS) to introduce epoxy groups (Fig. 5a) and with γ-aminopropyl triethoxysilane (ATES) to insert amino functionalities (Fig. 5b). However, these two immobilization strategies produced considerably less performing preparations.
Applications of RH-CaLB in solvent-free esterification and polycondensation
This preliminary screening of strategies for covalent immobilization induced to continue the testing of the RH-CaLB formulation also in esterification and polycondensation reactions. Industry makes large use of immobilized CaLB for the synthesis of an array of emollient esters for the cosmetic sector and these processes must prevent the leaching of the enzyme and the contamination of the product.2 Therefore, the robust anchoring of CaLB on functionalized RH might meet such technical requirements. RH-CaLB was employed in the esterification between lauric acid and propyl-laurate. As a reference, the reaction was also carried out with the CaLB preparation obtained by adsorption and crosslinking on PS-DVB resin (Diaion-CaLB; Table 2, entry 6), which resulted as the most active covalent preparation among those employing commercial resins.Since the distribution of water is crucial in esterification reactions because of the unfavorable thermodynamics, the effect of the hydration of the two reaction profiles was also monitored. Half of the immobilized preparations were stored as wet while the rest was dried (see Materials and methods section) and the behavior of the different formulations was compared. The reactions were carried out using the same percentage in weight of the biocatalysts and the water content of wet and dry preparations is reported in Table 6.
Interestingly, the profiles reported in Fig. 6 indicate that both wet and dry RH-CaLB reach the same conversion and equilibrium after 8 h, although initial rate of the dry preparation is lower. On the contrary, the two Diaion-CaLB preparations display similar initial rates but, as expected, the reaction catalyzed by the wet biocatalyst is affected by the competing hydrolytic reaction once the conversion approaches 50%. The reaction profiles suggest a distinct behavior of RH, which seems to retain the water within the matrix and prevent the release to the bulk medium.
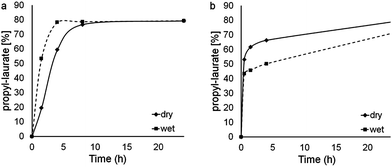 | ||
| Fig. 6 Effect of the dehydration of RH-CaLB (a) and Diaion-CaLB (b) on the reaction profile for the propyl-laurate synthesis. | ||
Indeed, a similar behavior had been previously described for porous silicates used as immobilization matrix.34 As reported above, at least 20% of RH is made by silicates and this observation suggest the need of further investigation addressing the microscopic organization of RH, its porosity and verify any ability to tightly bind water.
Recently we demonstrated that the covalent immobilization of enzymes on appropriate robust carriers is the pre-requisite for carrying out enzymatic polycondensations while preventing the detachment of the enzyme protein from the support.19,20,37
Regarding the improvement of the efficiency of the biocatalyst in solvent-free polycondensation, of course mono-molecular dispersion of the native enzyme would lead to the highest reaction rate but the contamination of product with the biocatalyst must be avoided. As an alternative solution, it was demonstrated that distributing the catalyst on the widest carrier surface facilitates the enzyme–substrate approach and promotes the polycondensation.19 CaLB covalently immobilized on carriers with low protein loading showed to be a practical and economical solution to the problem. Such solution is necessarily related to the possibility of using inexpensive, widely available and possibly renewable carriers. RH potentially meets all these requirements, supposing that efficient functionalization and immobilization methods are developed.
Therefore, the RH-CaLB formulation has been tested in solvent free polycondensation of dimethyl adipate with 1,4-butanediol. The reactions were carried out employing both the wet and the dry formulations at 70 °C using 10% w w−1 (respect to the whole amount of monomers) of biocatalyst in order to observe the effect of RH matrix on the distribution of water and, ultimately, the proceeding of the reaction. The conversion was determined by monitoring the 1H-NMR signal of the diester (see Materials and methods). After 24 h the reaction catalyzed by the dry RH-CaLB reached 42% of monomers conversion (see ESI, Fig. S8†) whereas in the case of wet RH-CaLB the conversion was quite similar, around 36% (see ESI, Fig. S9†). However, upon prolonged incubation (120 h) the reaction catalyzed by the dry formulation reached 49% of conversion whereas in the case of the wet RH-CaLB the conversion decreased and the equilibrium was set at about 25% because of the moisture contained in the system.
The final product was a viscous sticky colorless liquid, and the ESI-MS characterization indicated the formation of a mixture of oligomers between 3 and 9 units with a molecular weight in the range of 290–903 Da (Fig. 7), which is comparable to what observed in polycondensation catalyzed by Epox-CaLB (See ESI Fig. S6 and S7†) and previously observed with a different preparation of CaLB covalently immobilized on EC-EP carriers.37 It must be underlined that the conversion obtained with Epox-CaLB was higher (87%) but the reaction was carried out under reduced pressure (70 mmHg), which is demonstrated to affect polycondensation kinetics.19,20
Preliminary evaluation of the greenness of RH as enzyme carrier
Although a complete life cycle assessment of the methodologies here reported is beyond the scope of the study and our scientific competence, it is possible to perform a preliminary identification of the factors that are relevant in determining the greenness of RH as enzyme carrier. When considering the impact of a certain material, we have to consider both upstream and downstream environmental costs. Over 75% of the known and quantifiable impacts associated with fossil based plastic are located in the upstream portion of the supply chain across all sectors, where ‘upstream’ refers to impacts generated from the extraction of raw materials to the manufacturing of feedstock.38 This is also confirmed by the LCA studies addressing specifically methacrylic resins composed by glycidyl methacrylate and ethylene dimethyl acrylate.4‘Downstream’ refers to impacts generated once the product has been discarded by the consumer. Besides being fully renewable, at the end of its proposed applications, rice husk can still be used for for generating energy or in different manufacturing sectors in accordance with the circular economy principles. Of course, the disposal or recycling of RH will depend on the nature of the chemicals came in contacts with the immobilized biocatalyst. On that respect, application of RH carriers in food sector would enable its use as fertilizer, medium for gardening, ruminant feed or source of fiber in pet foods. Moreover, rice residues have been used as carbon source for anaerobic digestion in biogas and methane plants, cellulose source for bioethanol fermentation or simply burnt for energy production.39 Different routes for valorization of RH include the manufacture of brick, cement and insulation materials. Finally, due to large silica content in rice husk ash, extraction of high purity silica is economical.8
By analyzing the experimental protocols employed for immobilizing the enzymes, it is evident that the use of vegetable oil as medium for the in situ physical adsorption of CaLB represents an advantage in terms of avoiding the use of large volumes of buffer. This method results the most environmentally friendly since it requires no chemical derivatization of the matrix.
The covalent immobilization on activated RH is carried out under similar media as generally reported for epoxy- or amino-functionalized resins. Notably, data in Fig. 6 indicate that RH-CaLB can be employed without previous dehydration treatment. The latter is generally accomplished either by rinsing with water miscible solvents (e.g. acetone) or by energy intensive procedures (e.g. lyophilization, reduced pressure).
Concerning the chemicals used for the functionalization of RH, hexamethylenediamine is considered as harmful to aquatic invertebrates, but as it is readily biodegradable and not potentially bio-accumulative, it is not classified as dangerous for the environment according to EU regulation (EC) 1272/2008 (see ESI, Table S2 for full details†). Moreover, a bio-based routes for HMDA production has been recently reported, along with the corresponding life cycle assessment.40
The standard preparation of glutaraldehyde is performed from acetylene, ethanol and acrolein in a three-step process. However, methods for the production of bio-based glutaraldehyde have been patented.41 Ecotoxicological studies42 reported glutaraldehyde as acutely toxic to aquatic organisms but it is readily biodegradable in the freshwater environment and has the potential to biodegrade in the marine environment. Moreover, it has little tendency to bio-accumulate.
Among the chemicals used for RH functionalization, sodium periodate is probably the one bringing more concerns, due to its oxidizing action that can cause explosions and skin corrosion. It has a specific target toxicity on thymus gland and causes aquatic toxicity. However it is considered not bio-accumulative or very persistent (see ESI Table S2†).
It is important to mention that alternative sustainable routes for oxidizing cellulose have been already reported, which make use of laccase enzymes.43,44 Therefore, after this first study that demonstrates the applicability of functionalized RH the future investigations will pursue the objective of improving the sustainability of the oxidative step.
Materials and methods
Materials
Samples of rice husk (carnaroli type) were kindly donated by Riseria Cusaro S.r.L. (Binasco, Italy). Invertase from S. cerevisiae (ISc, batch IV031047, 2100 U mg−1 protein) was purchased from Sigma-Aldrich. ISc was provided in the form of powder and the enzyme solution used for immobilization had a concentration of 0.39 mg mL−1. Commercial Candida antarctica lipase B (CaLB) (batch LCN02115 with an activity of 6740 U mL−1) was purchased from Novozymes (DK). PROTEX 14L, batch 4861905794 (280 U mL−1 – casein assay) were from Sigma-Aldrich. The solvents were standard laboratory grade. Alcohol, organic acid, and other reagents were purchased either from Aldrich Chemical Co. (Milwaukee, WI) or Sigma-Aldrich and used as received if not otherwise specified. Diaion HP20-L and Sepabeads EC-EP/S were purchased from Resindion (Milano, Italy). Lewatit were purchased from Lenntech (The Netherlands).Characterization of the lignocellulosic supports
| Moisture content, % = [(A − B)/A] × 100 |
| Lignin, % = A × 100/W |
When the temperature of the distillate reached about 20 °C, a 3.85 N HCl solution was added up to 250 mL and the solution were mixed thoroughly. 5.0 mL of the distillate were transferred into a 50 mL volumetric flask, 25.0 mL of orcinol reagent were added mixing and the flask immersed in a water bath at 25 ± 1 °C.
After 60 min, ethanol was added up to the 50 mL mark, mixed, and the flask was returned to the water bath; then, after another 60 min, the absorbance of the solution was measured at 630 nm. The amount of xylan (mg) in the rice husk was calculated using the calibration graph.
| Pentosans, % = A/10W |
Calculate milligrams of xylan (anhydroxylose) in the specimen:
| Xylan (mg) = xylose (mg) × 0.88 |
Plot of data is available in ESI, Fig. S7.†
| Cellulose (%) = D × 100/C |
It must be noted that the percentages of components were calculated on the dried samples, and this explains why the total composition for RH in Table 1 exceeds 100%.
Enzymes activity assays
The acid value (AV) was calculated as follows:
| AV = (56.11 × CKOH × VKOH)/msample |
The yield of propyl-laurate was calculated as follows:
| PL (%) = (AV0 − AVsample) × 100/AV0 |
The amount of propyl-laurate was calculated as follows:
μmol PL = (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × % PL)/100 000 × % PL)/100 |
Enzymatic activity was calculated as follows:
| Activity (U gdry−1) = μmol PL/(mbiocatalyst × t) |
Enzymes immobilization procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U gdry−1) was added and the mixture was stirred for 20 h at 30 °C in a rotary wheel. In order to calculate the percentage of protein bonded on the support, the activity of supernatant was determined. In the case of immobilization in oil, 1.0 g dry rice husk, average particle size 200 μm (RH200), was weighted in 30 mL plastic syringe. 12 mL dry rapeseed oil (dried overnight under molecular sieves) was added together with 2.5 mL Lipozyme CaLB (16
000 U gdry−1) was added and the mixture was stirred for 20 h at 30 °C in a rotary wheel. In order to calculate the percentage of protein bonded on the support, the activity of supernatant was determined. In the case of immobilization in oil, 1.0 g dry rice husk, average particle size 200 μm (RH200), was weighted in 30 mL plastic syringe. 12 mL dry rapeseed oil (dried overnight under molecular sieves) was added together with 2.5 mL Lipozyme CaLB (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 U gdry−1). The mixture was allowed to stir at room temperature in a shaker at 250 rpm.
850 U gdry−1). The mixture was allowed to stir at room temperature in a shaker at 250 rpm.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U gdry support−1) or 15 mL ISc solution (2000 U gdry support−1) and was incubated at room temperature under blood rotation for 17 h. After enzyme adsorption procedure, cross-linking was achieved by the addition of 1.5 mL glutaraldehyde solution 3% w v−1. The process was allowed to proceed for 4 h at 25 °C under orbital shaking (200 rpm).
000 U gdry support−1) or 15 mL ISc solution (2000 U gdry support−1) and was incubated at room temperature under blood rotation for 17 h. After enzyme adsorption procedure, cross-linking was achieved by the addition of 1.5 mL glutaraldehyde solution 3% w v−1. The process was allowed to proceed for 4 h at 25 °C under orbital shaking (200 rpm).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U gdry−1) was added. 0.5 mL PEG-3350 solution (2 mg mL−1) in 0.05 M Nap pH 7.0 was added together with the enzyme solution and the coupling took place at 25 °C for 19 h. At the end of immobilization process, the preparation was washed 14 times with 1.2 mL 0.1 M Nap pH 7.0 (6 mL gdry support−1).
000 U gdry−1) was added. 0.5 mL PEG-3350 solution (2 mg mL−1) in 0.05 M Nap pH 7.0 was added together with the enzyme solution and the coupling took place at 25 °C for 19 h. At the end of immobilization process, the preparation was washed 14 times with 1.2 mL 0.1 M Nap pH 7.0 (6 mL gdry support−1).In the case of ISc, enzyme fixation was performed by placing the washed support in contact with 6.0 mL ISc solution (2000 U gdry support−1) in 0.1 M sodium acetate buffer (Naa), pH 4.5 at 25 °C and 200 rpm for 17 h. The preparation was washed 4 times (10 min) with 1.6 mL 0.05 M Nap pH 7.0 (8 mL gdry support−1) and 5 times (3 min) with 0.1 M Nap pH 7.0.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U gdry support−1. 0.5 mL PEG-3350 (2 mg mL−1) in 0.05 M Nap pH 7.0 was added together with the enzyme solution. The coupling took place at 25 °C for 21 h. After immobilization, the biocatalyst was separated by filtration and washed for several times with 0.05 M Nap pH 7.0 and 0.1 M Nap pH 7.0 (6 mL gdry support−1). The washing solutions have been monitored at 260 and 280 nm until the absorbance become constant. The biocatalyst was used as wet preparation.
000 U gdry support−1. 0.5 mL PEG-3350 (2 mg mL−1) in 0.05 M Nap pH 7.0 was added together with the enzyme solution. The coupling took place at 25 °C for 21 h. After immobilization, the biocatalyst was separated by filtration and washed for several times with 0.05 M Nap pH 7.0 and 0.1 M Nap pH 7.0 (6 mL gdry support−1). The washing solutions have been monitored at 260 and 280 nm until the absorbance become constant. The biocatalyst was used as wet preparation.Entry 13, Table 5: the amination was carried out by reacting oxidized rice husk with 40 mL hexamethylene diamine 0.9 M solution in methanol at 25 °C for 72 h. In the case of entry 16 Lipozyme CaLB L solution was added to give a loading of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U gdry support−1.
000 U gdry support−1.
Entry 14, Table 5: the amination was carried out by reacting oxidized rice husk with 40 mL ethylene diamine 0.9 M solution in methanol at 25 °C for 72 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U gdry−1) in 1 mL 0.5 M Nap pH 8.0 was used. The enzymatic solution has been corrected to pH 8.0 using NaOH 0.5 M. The coupling took place in a rotating wheel at 35 °C for 17 h. After immobilization, the biocatalyst was separated by filtration and washed for several times with Nap 0.02 M pH 8.0 (6 mL gdry support−1). The washing solutions have been monitored at 260 and 280 nm until the absorbance become constant. The biocatalyst was used as wet preparation.
000 U gdry−1) in 1 mL 0.5 M Nap pH 8.0 was used. The enzymatic solution has been corrected to pH 8.0 using NaOH 0.5 M. The coupling took place in a rotating wheel at 35 °C for 17 h. After immobilization, the biocatalyst was separated by filtration and washed for several times with Nap 0.02 M pH 8.0 (6 mL gdry support−1). The washing solutions have been monitored at 260 and 280 nm until the absorbance become constant. The biocatalyst was used as wet preparation.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U gdry−1) in 1 mL Nap 0.02 M pH 7.0 were used. After immobilization, the biocatalyst was separated by filtration and rinsed with Nap 0.02 M pH 7.0 (6 mL gdry support−1). The washing solutions have been monitored at 260 and 280 nm until the absorbance become constant. The biocatalyst was used as wet preparation.
000 U gdry−1) in 1 mL Nap 0.02 M pH 7.0 were used. After immobilization, the biocatalyst was separated by filtration and rinsed with Nap 0.02 M pH 7.0 (6 mL gdry support−1). The washing solutions have been monitored at 260 and 280 nm until the absorbance become constant. The biocatalyst was used as wet preparation.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U gdry support−1. The coupling took place at 25 °C for 22 h. The activity of supernatant has been tested after 22 h in order to determine the protein bounded to the support.
000 U gdry support−1. The coupling took place at 25 °C for 22 h. The activity of supernatant has been tested after 22 h in order to determine the protein bounded to the support.In the case of Diaion HP20L, the adsorbed enzyme was also crosslinked with 1 mL glutaraldehyde solution 3% w v−1 in Nap 0.1 M pH 8.0 took place in a rotating wheel for 4 h. After immobilization, the biocatalyst was separated by filtration and washed several times with Nap 0.05 M pH 7.0 and Nap 0.1 M pH 7.0 until the absorbance at 260 and 280 nm of the filtrates become constant. The water content of wet and dry preparation was determined by drying the samples at 120 °C for 6 h. The protein bounded on polystyrene resin was 65%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U (TBU) per dry gram of support of Lipozyme CALB L, previously diluted in Nap 0.5 M pH 8.0, was added to the polymeric support to obtain a support/buffer ratio equal to 1 g per 4 mL. Immobilization was carried out at 25 °C for 24 h. When required, after immobilization the immobilized enzyme was dried by washing with acetone (3 × 2 mL per g of carrier) on a Buchner filter and the excess of acetone was removed leaving the biocatalyst on the filter under reduced pressure overnight.
000 U (TBU) per dry gram of support of Lipozyme CALB L, previously diluted in Nap 0.5 M pH 8.0, was added to the polymeric support to obtain a support/buffer ratio equal to 1 g per 4 mL. Immobilization was carried out at 25 °C for 24 h. When required, after immobilization the immobilized enzyme was dried by washing with acetone (3 × 2 mL per g of carrier) on a Buchner filter and the excess of acetone was removed leaving the biocatalyst on the filter under reduced pressure overnight.Protease activity assay (casein hydrolysis)
Hydrolytic activity of protease has been determined using casein as substrate, at 40 °C and pH 6.5. The reaction was made up of 5 mL casein 2% w v−1 and 1 mL of enzyme solution in phosphate buffer 0.1 M pH 6.5 (1.19 mg protein or 100 mg wet immobilized preparation). The reaction was carried out at 40 °C for 15 min and stopped with 5 mL trichloroacetic acid. The absorbance of the supernatant was measured at 280 nm. One unit will hydrolyze casein to produce peptide equivalent to 1.0 μmol (181 μg) of tyrosine per minute at pH 6.5 and 40 °C.Computational construction of 3D models and surface analysis
Protein structures were visualized and recorded using the PyMOL software. The 3D-structures used for the hydrophobicity comparisons were retrieved from the PDB with the codes 1TCA for CaLB, 5DPF for Thermolysin and 4EQV for Invertase.45The representation and the calculation of the hydrophobic enzyme surfaces were performed by GRID mapping, using two different probes describing and quantifying different interactions: WATER (dipolar interactions and hydrogen bond formation), DRY (hydrophobic interactions).25
Hydrolytic activity assay for ISc
ISc activity was determined using the method of described by Bryjak et al.,46 incubating 0.1 mL of enzyme solution (0.002 mg mL−1) or 3 mg wet immobilized preparation with 0.9 mL of sucrose 1% w v−1 in 0.1 M Naa (pH 4.5) at 55 °C for 20 min. 0.1 mL were removed from the reaction mixture and placed in a separate tube containing 2.9 mL p-hydroxybenzhydrazide solution 0.5% w v−1 in a 0.5 M NaOH aqueous solution. The assay was based on the formation of oxazones from reducing sugars and p-hydroxybenzhydrazide. To stop the reaction, the reaction mixture was heated for 15 min in a boiling water bath. Finally, the absorbance was read at 410 nm. One unit of ISc is defined as the amount of enzyme, which liberates 1 μmol of glucose per minute per mL under the given assay conditions.Drying of RH-CaLB and Diaion-CaLB
One part of immobilized preparations was stored as wet at 4 °C, and another part was washed 2 times with acetone (1 gwet support/2 mL acetone) and dried for 30 min under vacuum (750 mbar), followed by drying for 24 h at room temperature on a filter paper. The water content of wet and dry preparations was determined by drying the samples at 120 °C for 6 h in an oven.Solvent-free enzymatic polyesterification catalyzed by RH-CaLB
10% w w−1 (respect to the whole amount of monomers) of RH-CaLB (activity of 2178 U gdry−1 preparation) were added to 5 mmol of DMA and 5 mmol of BDO previously mixed together. The reaction was conducted at 70 °C at atmospheric pressure on a thin-film system, as previously described.19,20,37 The final product was a viscous sticky colorless liquid, which was solubilized in DCM. After solvent evaporation, the crude product was analyzed by ESI-MS and 1H-NMR without any further purification.Polycondensation of DMA with BDO catalyzed by Epox-CaLB
The DMA (35 mmol) and the diol (BDO, 35.8 mmol) were mixed in a round bottom flask and the biocatalyst, 10% w w−1 with respect to the total amount of monomers, Epox-CaLB (activity of about 2000 U gdry−1) was added. The reaction was carried out for 24 h at 50 °C on thin film at reduced pressure (70 mmHg).1H-NMR spectra related to polycondensation of DMA and BDO
Spectra were recorded on an JEOL Ex-270 spectrometer operating (270 MHz). The solvent was CDCl3 if not otherwise specified.Original spectra are reported in ESI.† The conversion of the reactions was monitored by exploiting the 1H-NMR) of the methoxy groups of the unreacted diester at ∼3.6 ppm and the signals at δ = 2.24 of methylene protons (–CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–C(O)O–, the latter assumed constant throughout the reaction. The formation of the oligomers is confirmed by the signals of protons of methylene group adjacent to ester moiety at gives signals in the range of ∼3.8–4.0 ppm. Protons of methylenic protons of DMA give signals at 1.58 ppm (see ESI, Fig. S6†).
2–C(O)O–, the latter assumed constant throughout the reaction. The formation of the oligomers is confirmed by the signals of protons of methylene group adjacent to ester moiety at gives signals in the range of ∼3.8–4.0 ppm. Protons of methylenic protons of DMA give signals at 1.58 ppm (see ESI, Fig. S6†).
Thin layer chromatography (TLC)
For the TLC analysis on silica gel, glass plates of dimensions 20 × 20 cm (Macherey-Nagel) were used. The spots were visualized by treating the silica plates with a solution mixture of KMnO4/KOH (1.25/0.5%). The components were separated employing ethyl acetate as mobile phase.Electrospray ionization mass spectrometry (ESI-MS)
The crude reaction mixtures were analyzed on Esquire 4000 ESI-MS ion trap Bruker (Karlsruhe, Germany) instrument electrospray positive ionization by generating the ions in an acidic environment. Around 10 mg of sample was dissolved in 1 mL methanol containing 0.1% v v−1 formic acid. The generated ions were positively charged with m/z ratio falls in the range of 100–1000. The subsequent process of deconvolution allows the reconstruction of the mass peaks of the chemical species derived from the analysis of the peaks generated.Conclusions
This study reports a first overview on the potential of an inexpensive, although chemically complex and mechanically resistant, biomaterial namely rice husk. Further investigations will be necessary for optimizing immobilization protocols and especially for improving the greenness of the functionalization procedures, more specifically through, benign enzymatic oxidative routes.43,44The data here reported indicate that there is potential for moving towards a new paradigm in enzyme immobilization, and replace MA and PS resins with materials that meet better the cost and environmental requirements of biobased and chemical industry.
Keeping in mind that 10% of the bulk chemical market of today, being about 330 Mton per year, will be manufactured using immobilized enzymes in the next few decades at a cost of 100 US $ per ton, this would mean a turnover of 3.3 billion US $1. In addition to this, the use of immobilized enzymes in the biodiesel industry will likely also grow, which will thus add significant potential to the total of the enzyme market, which is around 4 billion US $ today. As sustainability is already an issue, more solvent-free synthesis will become the rule. Most solvent-free viscous systems cited above suffer from mass transfer limitations, which means that using a large number of enzymatic units concentrated in a small volume would turn into low process efficiency. For this reason, the present study aims aim at developing cheap biocatalysts consisting of less enzymatic units “diluted” on a wider surface of cheap carriers.
For most non-aqueous systems, such as biodiesel, covalent coupling is not required, with corresponding procedure simplification and costs reduction. For other applications, such as cosmetic and food sectors, contamination of enzymes represents an issue. On that respect, the most fascinating aspect of rice husk is represented by the chemical variety and complexity of its lignocellulosic constituents, which make this material prone to multiple and benign enzymatic modifications aiming at tuning hydrophilic–hydrophobic properties, inserting extra chemical functionalities or grafting moieties.
Acknowledgements
We thank Paolo Cusaro and Riseria Cusaro S.r.l. (Binasco, Italy) for providing the rice husk and for the useful discussions. We acknowledge VERDER SCIENTIFIC S.r.l. (Bergamo, Italy) for milling the rice husk. Valerio Ferrario is grateful to MIUR (Ministero dell'Istruzione, dell'Università e della Ricerca – Roma) and to Università degli Studi di Trieste for financial support. This project (Livia Corici) has received funding from the European Union's Seventh Framework Programme for research, technological development and demonstration under grant agreement No. 289253 (REFINE project).References
- R. DiCosimo, J. McAuliffe, A. J. Pouloseb and G. Bohlmann, Chem. Soc. Rev., 2013, 42, 6437–6474 RSC.
- M. B. Ansorge-Schumacher and O. Thum, Chem. Soc. Rev., 2013, 42, 6475–6490 RSC.
- M. C. Franssen, P. Steunenberg, E. L. Scott, H. Zuilhofac and J. P. Sanders, Chem. Soc. Rev., 2013, 42, 6491–6533 RSC.
- S. Kim, C. Jimenez-Gonzalez and B. E. Dale, Int. J. Life Cycle Assess., 2009, 14, 392–400 CrossRef CAS.
- A. Pellis, E. Herrero Acero, V. Ferrario, D. Ribitsch, G. M. Guebitz and L. Gardossi, Trends Biotechnol., 2016, 34, 316–328 CrossRef CAS PubMed.
- C. O. Tuck, E. Pérez, I. T. Horváth, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695–699 CrossRef CAS PubMed.
- N. K. Sharma, W. S. Williams and A. Zangvil, J. Am. Ceram. Soc., 1984, 67, 715–720 CrossRef CAS.
- D. Yang, T. Fan, H. Zhou, J. Ding and D. Zhang, PLoS One, 2011, 6, e24788 CAS.
- S. Ajitha, Ordered mesoporous silica as supports for immobilization of biocatalyst, PhD thesis, 2012.
- D. P. Wong, R. Suriyaprabha, R. Yuvakumar, V. Rajendran, Y.-T. Chen, B.-J. Hwang, L.-C. Chen and K.-H. Chen, J. Mater. Chem. A, 2014, 2, 13437–13441 CAS.
- S. Tabata, H. Iida, T. Horie and S. Yamada, Med. Chem. Commun., 2010, 1, 136–138 RSC.
- K. M. Hello, M. J. Mohammed, A. M. Yasser, F. Adam and Z. Farag, J. Catal., 2014, 1–9 Search PubMed.
- F. O. Nwosu, B. I. Olu-Owolabi, K. O. Adebowale, T. Henle and U. Schwarzenbolz, Res. J. Appl. Sci., 2011, 6, 501–511 CrossRef.
- F. N. Sadon, A. S. Ibrahem and K. N. Ismail, J. Purity, Util. React. Environ., 2012, 1, 308–334 CAS.
- M. G. Jackson, Anim. Feed Sci. Technol., 1977, 2, 105–130 CrossRef CAS.
- S. Chandrasekhar, K. G. Satyanarayana, P. N. Pramada, P. Raghavan and T. N. Gupta, J. Mater. Sci., 2003, 38, 3159–3186 CrossRef CAS.
- R. Bergamasco, F. J. Bassetti, F. F. Moraes and G. M. Zanin, Braz. J. Chem. Eng., 2000, 17, 873–880 CrossRef CAS.
- S. Cantone, V. Ferrario, L. Corici, C. Ebert, D. Fattor, P. Spizzo and L. Gardossi, Chem. Soc. Rev., 2013, 42, 6262–6276 RSC.
- L. Corici, A. Pellis, V. Ferrario, C. Ebert, S. Cantone and L. Gardossi, Adv. Synth. Catal., 2015, 357, 1763–1774 CrossRef CAS.
- A. Pellis, L. Corici, L. Sinigoi, N. D'Amelio, D. Fattor, V. Ferrario, C. Ebert and L. Gardossi, Green Chem., 2015, 17, 1756–1766 RSC.
- V. Ferrario, H. Veny, E. De Angelis, L. Navarini, C. Ebert and L. Gardossi, Biomolecules, 2013, 3, 514–534 CrossRef PubMed.
- S. M. Kotwal and V. Shankar, Biotechnol. Adv., 2009, 27, 311–322 CrossRef CAS PubMed.
- J. Otte, S. M. A. Shalaby, M. Zakora and M. S. Nielsen, Int. Dairy J., 2007, 17, 1460–1472 CrossRef CAS.
- A. Basso, P. Braiuca, S. Cantone, C. Ebert, P. Linda, P. Spizzo, P. Caimi, U. Hanefeld, G. Degrassi and L. Gardossi, Adv. Synth. Catal., 2007, 349, 877–886 CrossRef CAS.
- V. Ferrario, C. Ebert, L. Knapic, D. Fattor, A. Basso, P. Spizzo and L. Gardossi, Adv. Synth. Catal., 2011, 353, 2466–2480 CrossRef CAS.
- S. F. D'Souza and S. S. Godbole, J. Biochem. Biophys. Methods, 2002, 52, 59–62 CrossRef.
- K. Nakanishi, A. Takeuchi and R. Matsuno, Appl. Microbiol. Biotechnol., 1990, 32, 633–636 CrossRef CAS PubMed.
- F. Chen, F. Zhang, F. Du, A. Wang, W. Gao, Q. Wang, X. Yin and T. Xie, Bioresour. Technol., 2012, 115, 158–163 CrossRef CAS PubMed.
- P. Monsan, Enzymes immobilized on a solid support containing cellulose and lignin, US Pat., 4405715, 1983.
- Surface Science Techniques, ed. G. Bracco and B. Holst, Springer Series in Surface Sciences, Springer-Verlag, Berlin Heidelberg, 2013, vol. 51 Search PubMed.
- V. H. Perez, G. S. da Silva, F. M. Gomes and H. F. de Castro, Biochem. Eng. J., 2007, 34, 13–19 CrossRef CAS.
- A. I. S. Brígda, Á. D. T. Pinheiro, A. L. O. Ferreira, G. A. S. Pinto and L. R. B. Gonçalves, Appl. Biochem. Biotechnol., 2007, 136–140, 67–80 CrossRef PubMed.
- L. Gardossi, L. Sinigoi, P. Spizzo and D. Fattor, Method for covalent immobilization of enzymes on functionalized solid polymeric supports, WO2012085206 A1, 2012.
- A. Basso, L. De Martin, C. Ebert, L. Gardossi, P. Linda and V. Zlatev, J. Mol. Catal. B: Enzym., 2001, 11, 851–855 CrossRef CAS.
- J.-F. Stumbé and B. Brunchmann, Macromol. Rapid Commun., 2004, 25, 921–924 CrossRef.
- A. Pellis, E. Herrero Acero, L. Gardossi, V. Ferrario and G. M. Guebitz, Polym. Int., 2016, 65, 861–871 CrossRef CAS.
- A. Pellis, V. Ferrario, B. Zartl, M. Brandauer, C. Gamerith, E. Herrero Acero, C. Ebert, L. Gardossi and G. M. Guebitz, Catal. Sci. Technol., 2016, 6, 3430–3442 CAS.
- J. Raynaud, Valuing Plastics: The Business Case for Measuring, Managing and Disclosing Plastic Use in the Consumer Goods Industry, ed. J. Richens and A. Russell, Copyright: United Nations Environment Programme (UNEP), 2014 Search PubMed.
- S. Kumar, P. Sangwan, R. Dhankhar, V. Mor and S. Bidra, Res. J. Chem. Environ. Sci., 2013, 1, 126–129 CAS.
- A. B. Dros, O. Larue, A. Reimond, F. De Campo and M. Pera-Titus, Green Chem., 2015, 17, 4760–4772 RSC.
- J. L. Couturier and J. L. Dubois, US Pat., 8779213 B2, Arkema, France, 2014.
- H. W. Leung, Ecotoxicol. Environ. Saf., 2001, 49, 26–39 CrossRef CAS PubMed.
- S. Riva, Trends Biotechnol., 2006, 24, 219–226 CrossRef CAS PubMed.
- E. Aracri, C. Valls and T. Vidal, Carbohydr. Polym., 2012, 88, 830–837 CrossRef CAS.
- M. A. Sainz-Polo, M. Ramírez-Escudero, A. Lafraya, B. Gonzalez, J. Marín-Navarro, J. Polaina and J. Sanz-Aparicio, J. Biol. Chem., 2013, 288, 9755–9766 CrossRef CAS PubMed.
- J. Bryjak, J. Liesiene and V. Stefuca, Cellulose, 2008, 15, 631–640 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12065b |
| ‡ Current address: Institute of Chemistry Timisoara of Romanian Academy, Mihai Viteazul 24, 300223 Timisoara, Romania. |
| § Current address: University of Natural Resources and Life Sciences, Vienna, Department for Agrobiotechnology IFA-Tulln, Institute for Environmental Biotechnology, Konrad Lorenz Strasse 20, A-3430 Tulln an der Donau, Austria. |
| ¶ Current address: Università degli Studi di Trieste, Piazzale Europa 1, 34127, Trieste, Italy. |
| This journal is © The Royal Society of Chemistry 2016 |




