Synthesis and hydrogenation of anatase TiO2 microspheres composed of porous single crystals for significantly improved photocatalytic activity†
Abstract
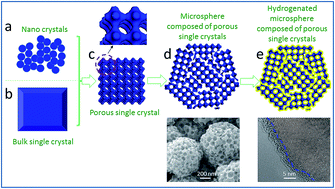
Anatase microspheres composed of porous single crystals were successfully synthesized via a facile route without preseeding treatment and then further modified using a surface hydrogenation process. The porous materials obtained exhibit excellent photocatalytic activity and good recyclability leading to great potential in practical applications.

 Please wait while we load your content...
Please wait while we load your content...