A reaction of 1,2-diamines and aldehydes with silyl cyanide as cyanide pronucleophile to access 2-aminopyrazines and 2-aminoquinoxalines†
Abstract
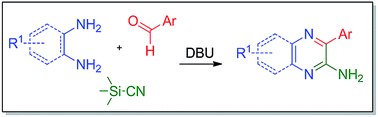
A new condensation reaction of ethylene-1,2-diamines or o-phenylenediamines and aromatic aldehydes with TMSCN as a cyanide-pronucleophile is documented. The reaction proceeds through a tandem sequence of desilylation, Strecker reaction, amidine-forming cyclization and dehydrogenative aromatization, and provides a straightforward synthetic route to access synthetically and biologically important motifs, 3-aryl substituted 2-aminopyrazines and 2-aminoquinoxalines. DBU with its unique function and rate-accelerating effect has made it possible to realize a reaction that involves several C–C/N/Si bond forming/breaking events. Interestingly, the protocol has enabled the desired tandem pathway, switching exclusively from usual transformations.

 Please wait while we load your content...
Please wait while we load your content...