Ultrathin nickel sulfide nano-flames as an electrode for high performance supercapacitor; comparison of symmetric FSS-SCs and electrochemical SCs device
Abstract
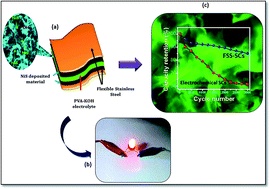
Metal sulfides have received well deserved attention due to their excellent electrical conductivity and thermal stability, as compared to metal oxides, allowing them to achieve a high capacitance and energy density for portable energy storage devices. In this study, the preparation of highly porous nano-flames composed of nickel sulfide (NiS) thin film on a cost effective, flexible stainless steel substrate through a trouble free, inexpensive and simple chemical bath deposition (CBD) method is reported. The prepared nano-flames composed of a NiS thin film demonstrates the excellent electrochemical features with a maximum specific capacitance (Cs) of 750.6 F g−1 at a scan rate of 5 mV s−1 in a three electrode system. Furthermore, the portable symmetric flexible solid state supercapacitor (FSS-SC) and electrochemical supercapacitor (SC) are fabricated and tested. In comparison with the symmetric electrochemical SC, the symmetric FSS-SC shows an excellent electrochemical performance with a high Cs of 104 F g−1 at 5 mV s−1 with a good electrochemical stability of 85.3% over 3000 CV cycles. This study constitutes the first comparison of symmetric FSS-SCs and electrochemical SCs formed with NiS nano-flames. Such an impressive symmetric FSS-SC is predicted to be an exceptionally promising candidate for energy storage systems.

 Please wait while we load your content...
Please wait while we load your content...