Synthesis and multistate characterization of bis-flavylium dications – symmetric resorcinol- and phloroglucinol-type derivatives as stochastic systems†
Abstract
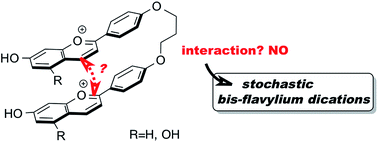
Two symmetric bis-flavylium dications were readily synthesized and further evaluated for their multistate profile. Both systems were shown to be fully stochastic and behave like a single compound suggesting that the two flavylium moieties do not communicate via the bridge linking them. Global pKa values of ca. 4 regarding the acid–base reaction between flavylium cation and quinoidal base were calculated using a stopped flow technique. It was further demonstrated that the equilibrium between AH+–AH+ and indistinguishable flavylium-quinoidal base isomers AH+–A (A–AH+) can be calculated by subtracting 0.3 pH units from the observed acid–base constant. On the other hand, the equilibrium between both flavylium-quinoidal base isomers and the bis-quinoidal base A–A is obtained by adding 0.3 pH units. Moreover, both systems do not have a cis–trans isomerization barrier and the rate constants of interconversion between flavylium cation and quinoidal base as a function of pH are fitted with a mono-exponential and follow a bell-shaped curve. The systems proved also to be photochromic and from the fitting of the bell-shaped curve, flash photolysis measurements and quantum yields, it is possible to calculate all rate and equilibrium constants and construct a global energy-level diagram. It was also proved that the rate constant to form both isomers AH+–Ct from the bis-flavylium upon a pH jump from 1 to less acidic solutions is twice that of the observed value and the formation of the bis-trans-chalcone from both isomers AH+–Ct is equal to the observed rate constant. An energy-level diagram of all the multistate species was constructed from the equilibrium constants.

 Please wait while we load your content...
Please wait while we load your content...