Ivy leaves extract based – lipid nanocarriers and their bioefficacy on antioxidant and antitumor activities
I. Lacatusua,
N. Badea*a,
G. Badeaa,
L. Brasoveanub,
R. Stana,
C. Otta,
O. Opreaa and
A. Megheaa
aFaculty of Applied Chemistry and Materials Science, University Politehnica of Bucharest, Polizu Street No. 1, 011061, Bucharest, Romania
bRomanian Academy, Virology Institute “Stefan S. Nicolau”, Mihai Bravu Street No. 285, 030304, Bucharest, Romania. E-mail: nicoleta.badea@gmail.com
First published on 3rd August 2016
Abstract
In this study, two issues commonly associated with phytochemical based nanotechnology were addressed: (1) the use of active compounds from medicinal herbs as functional ingredients entrapped into lipid-based nanocarriers; (2) the safety and efficacy of phytochemical-based nanocarriers with promising antioxidant and antitumor benefits. In this context, nanostructured lipid carriers (NLC) based on raspberry seed oil (Rso), pomegranate seed oil (Pso) and rice bran oil (Rbo) were synthesized and proven to be highly effective for the entrapment of hydrophilic ivy leaves extract (Ile). The size, morphology, zeta potential, entrapment efficiency and crystallinity of the Ile-loaded NLC were characterized. The effectiveness of Ile loaded-NLC has been proved by identifying a high capacity to scavenge free oxygen radicals (i.e. between 94 and 98%). 200 μg mL−1 Ile-NLC based on Rbo showed no significant effects on cellular viability in the fibroblast L929 cell line, while the tumor B16 cell line was markedly affected. MTS, RTCA assays and apoptosis examination revealed that these nanocarriers induce cytotoxicity and apoptosis in the murine melanoma B16 cell line. This study reports the first evidence of the association of hydrophilic and lipophilic phytochemicals in the same lipid nanocarriers, which appears to be a promising antioxidant and antitumor approach deserving further investigation.
1. Introduction
Medical and epidemiological research has shown that a high dietary intake of fruits and vegetables is strongly associated with a reduced risk of chronic diseases such as cardiovascular disease or cancer.1,2 Despite the pharmacological relevance of vegetable oils and herbal extracts, the biological potential of many plant active ingredients is still insufficiently explored in the nanotechnology field with implications in health associated industries. Given the worldwide current trends, the use of plant resources for the supply of active naturally-derived ingredients that manifest low side effects as well as multiple health benefits, opens up new perspectives in the biomedical field. Only a few studies have been conducted to fully or partially replace the nano-encapsulation of synthetic drugs with phytochemicals that may help to improve the therapeutic effects or to provide additional biological properties.3,4 The latest phytochemicals encapsulation studies were focused on the suitability of various nanostructured systems, such as phospholipid nanoparticles,5 double-layered multiple emulsions6 and nano-emulsions7 to efficiently entrap herbal extract (e.g. elderberry extract, saffron extract, olive leaf extract) in order to enhance the functionalities of phytochemicals.Nanostructured lipid carriers (NLC) derived from solid lipid nanoparticles (SLN) by replacing appropriate amounts of solid lipids by liquid lipids have demonstrated their great potential to serve as an efficient carrier system for active compounds of foods, cosmetic and pharmaceutical interests.8–11 The purpose of NLC-formulations is to produce particles in which the oil incorporated into the lipid core creates a less ordered solid lipid matrix and allows higher drug loading capacity and a lower possibility of drug expulsion during storage in comparison with SLNs.12,13 Over the past several years, the synthesis of NLC employing vegetable – derived oils was reported by several research groups,14,15 including our group. Compared to conventional NLC, vegetable oil – mediated synthesis of lipid nanocarriers is safe, effective and most importantly is endowed with a variety of biological activities such as antioxidant, photoprotective or antitumor activities.16,17
Despite the broad benefits of both natural entities, i.e. vegetable oils and phytochemicals, there is a lack in literature about their association with the solid lipid carriers. There are limited reports on the production of SLN employing plant-derived compounds such as Calendula officinalis extract-loaded SLN18 that improved the epithelium repair activity in the conjunctival WKD cell line, synthesis of Salvia officinalis or Satureja montana herbs loaded into SLN19 with high stability during small intestine digestion and curcumin-SLNs20 that may effectively reduce the expression of serum pro-inflammatory cytokines. Regarding the exploitation of NLC systems for the encapsulation of plant extract, it debuted only two years ago with a study of Oliveira et al. which describes the entrapment of eugenol rich clove extract into NLC in order to improve the nutraceutical features of natural antioxidants.21 The efficacy of lipid nanocarriers based on fish oils for entrapment of willow bark extract22 and of NLCs prepared with herbal oils (e.g. thistle oil, safflower oil, sea buckthorn oil) and loaded with lipophil carrot extract23 have been demonstrated in previous works of authors. To the best of our knowledge the combination of hydrophilic ivy leaves extract with NLC formulations was never done before. As result, the development of NLC than can host both water – and lipid – soluble phytochemicals (e.g. herbal extract and vegetable oil) represents a real chance to become one of the most promising approach that combine the commercially marketed and modern herbal nanomedicines. This study describes the production of NLC loaded with Ivy leaves extract (Ile) and prepared using different ionic and non-ionic emulsifier compositions and three lipid matrices based on raspberry seed oil (Rso), rice bran oil (Rbo) and pomegranate seed oil (Pso). Given the advantage of phytochemicals and the need to develop innovative strategies in the medical field, the aim of this work was to compare the suitability of co-loading vegetable oils and hydrophilic herbal extract into the same nanocarrier for cumulating the main properties of both natural actives that result in synergistic and complementary biological effects.
Ivy leaves extract, Ile (Hedera helix L.) has been used in traditional medicine for the treatment of respiratory disorders because of its expectorant and bronchospasmolytic effects, and numerous studies have been conducted to reveal its mechanism of action, safety and pharmacological effect.24–26 The chemical constituents of ivy leaves belong mainly to the natural classes of triterpene saponins, flavonoids and phenolic acids.27 Ivy leaves extract and its medicinal products are standardized based on the content of Hederacoside C (Fig. 1), which is one of the main active ingredients in Ile.
In addition to biological actions of Ile, vegetable oils selected in this study are known for their physiological and health-promoting role of fatty acids and several anti-oxidant, anti-inflammatory, anti-carcinogenic substances, such as carotenoids, flavonoids, phytosterols etc., especially in preventing cardiovascular diseases.28,29 Pso comprises a unique component, punicic acid, a conjugated polyunsaturated fatty acid considered as one of the strongest natural antioxidants.30 Some studies have revealed the potential effect of Pso on inflammatory diseases and treatment of neurodegenerative diseases.31,32 The phytochemical compounds from Rbo have been also responsible for therapeutic effects, such as antioxidant, photoprotective and hypoglycemic.33–35
2. Experimental
2.1. Materials
Polyoxyethylenesorbitan monolaurate (Tween 20) and sodium cholate were purchased from Merck (Germany). Synperonic PE/F68 (Poloxamer 188) and L-α-phosphatidylcholine were obtained from Sigma Aldrich Chemie GmbH (Munich, Germany). The solid lipids, glycerol monostearate and cetyl palmitate were obtained from Cognis GmbH (Germany) and Acros Organics (USA), respectively. The ivy leaves extract (Ile) with a content of 14.65% Hederacoside C (Hed C) was purchased from Finzelberg, Germany. Tris[hydroxymethyl] aminomethane, 5-amino-2,3-dihydro-1,4-phthalazinedione (luminol) were purchased from Sigma Aldrich Chemie GmbH and hydrogen peroxide was obtained from Merck (Germany).The vegetable oils were purchased from Elemental Company (Romania) and the fatty acids content has been determined by gas chromatography (gas-chromatograph Agilent Technologies, 7890 A, with MS detection), after a derivatization stage. The separation was done on Supelco SPTMI 2560 capillary column (100 m length, 0.25 mm diameter and 0.2 μm film thickness); the set temperature: from 140 °C (5 min) to 240 °C (10 min); He carrier gas (flow rate of 20 cm s−1); MS-detector; injection sample volume of 1 μL. The main components of vegetable oils, determined by GC-MS are presented in Table 1.
| Samplea | Vegetable oils and its main components | Ivy leaves extract (Ile), % | |
|---|---|---|---|
| a NLC 1-vegetable oil formulations were prepared with Tween 20 as main non-ionic surfactant (1.75%), while NLC 2-vegetable oil were synthesized by using sodium cholate as main ionic surfactant (2%). | |||
| NLC 1-Rso-Ile 1 | 3% raspberry seed oil | 53.66% linoleic acid | 0.5 |
| NLC 2-Rso-Ile 1 | 26.02% linolenic acid | 0.5 | |
| NLC 1-Rso-Ile 2 | 12.61% oleic acid | 1 | |
| NLC 2-Rso-Ile 2 | 3.07% γ-linolenic acid | 1 | |
| NLC 1-Rso | 2.50% palmitic acid | — | |
| NLC 2-Rso | 1.15% eicosenoic | ||
| 0.7% stearic acid | — | ||
| 0.29% arachidic acid | |||
| NLC 1-Pso-Ile 1 | 3% pomegranate seed oil | 71.1% punicic acid | 0.5 |
| NLC 2-Pso-Ile 1 | 8.4% linoleic acid | 0.5 | |
| NLC 1-Pso-Ile 2 | 7.9% oleic acid | ||
| NLC 2-Pso-Ile 2 | 4.8% palmitic acid | 1 | |
| NLC 1-Pso | 3.6% stearic acid | 1 | |
| NLC 2-Pso | 1.3% eicosenoic acid | — | |
| 0.8% arachidic acid | — | ||
| 0.3% behenic acid | |||
| 0.1% lignoceric acid | |||
| NLC 1-Rbo-Ile 1 | 3% rice bran oil | 43.99% oleic acid | 0.5 |
| 34.18% linoleic acid | 0.5 | ||
| 16.03% palmitic acid | 1 | ||
| 1.94% stearic acid | 1 | ||
| NLC 2-Rbo-Ile 1 | 1.25% linolenic acid | — | |
| NLC 1-Rbo-Ile 2 | 0.73% arachidic acid | — | |
| NLC 2-Rbo-Ile 2 | 0.63% eicosenoic acid | ||
| NLC 1-Rbo | 0.35% lignoceric acid | ||
| NLC 2-Rbo | 0.22% behenic acid | ||
2.2. Preparation of lipid nanocarriers loaded with ivy leaves extract
All free- and Ile-loaded lipid nanocarriers were produced by high pressure homogenization (HPH), using APV 2000 Lab Homogenizer, Germany. The conditions used for the preparation of NLCs containing different concentrations of ivy leaves extract were adapted from previously works of authors.36,37 The lipid formulations contain 10% (w/w) lipids mixture (Rso/Pso/Rbo, glycerol monostearate and cetyl palmitate) and 2.5% (w/w) surfactants mixture (Tween 20: L-α-phosphatidylcholine: Synperonic PE/F68 or sodium cholate: Tween 20: Synperonic PE/F68). Hot emulsions were obtained by adding, under stirring, the surfactant aqueous solution to the melted lipid phase, at around 80 °C. The formed hot emulsions were maintained under stirring for 15 min, and then were homogenized by applying 1613 RCF for 1 min (High-shear homogenizer PRO250, Germany) and further subjected to six homogenization cycles at 500 bar (HPH, APV 2000 Lab Homogenizer, Germany). The hot o/w nanoemulsions were cooled at room temperature to allow the recrystallization of lipid phase to form free- and Ile-loaded NLCs (Table 1). The aqueous dispersions of NLCs were frozen at −25 °C overnight and were lyophilized for 72 h, at −55 °C using an Alpha 1-2 LD Freeze Drying System Germany.2.3. Particle size determination
The mean hydrodynamic diameter (Zave) and the distribution width (PdI) of the Ile loaded lipid nanocarriers were determined by photon correlation spectroscopy (Zetasizer Nano ZS, Malvern Instrument Ltd., United Kingdom), which utilizes Dynamic Light Scattering to measure particle size. The DLS measures the diffusion of particles moving under Brownian motion and convert it to size and size distribution using the Stokes–Einstein relationship. Before measurements, the dispersions were diluted with deionized water to an adequate scattering intensity. Zave and PdI were given as average of three individual measurements.2.4. Zeta potential measurements
The zeta potential values were measured by using the principles of laser Doppler velocimetry. An electric field is applied to the aqueous dispersion of Ile-lipid nanocarriers which than move with a specific velocity. This velocity is measured using a laser interferometry technique and it is used for the calculation of the electrophoretic mobility (based on Helmholtz–Smoluchowsky equation) and from this the zeta potential value and zeta potential distribution. For the measurements, a Zetasizer Nano ZS, Malvern Instruments Ltd., U.K. equipped with He–Ne laser and λ = 633 nm was used. Each sample was dispersed in deionized water and was adjusted with 0.9% NaCl solution, in order to reach a conductivity of 50 μS cm−1. All measurements were performed at 25 °C, in triplicate and the mean value was reported.2.5. TEM analysis
The morphology of Ile-loaded NLC was observed by transmission electron microscopy (300 kV Tecnai G2 F30 S-TWIN microscope equipped with HAADF detector, EDX, EELS, Eindoven, Netherlands). The lipid nanocarriers were diluted with water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, v/v) before examination. Samples were prepared by depositing three drops of the nanocarrier aqueous suspension on the surface of a copper grid. The analysis was performed after one week of deposition.
100, v/v) before examination. Samples were prepared by depositing three drops of the nanocarrier aqueous suspension on the surface of a copper grid. The analysis was performed after one week of deposition.
2.6. Differential scanning calorimetry (DSC) analysis
Thermal analysis of the lyophilized free- and Ile loaded-lipid nanocarriers was performed in order to investigate the changes in the crystalline state of the samples. The study was performed by DSC using a differential scanning calorimeter Jupiter, STA 449C (Netzsch, Germany). The samples were weighed (10 mg) directly in aluminum pans and scanned between 25 °C and 100 °C at a heating rate of 5 °C min−1. An empty aluminum pan was used as reference. The onset temperature, melting point (peak maximum), and melting enthalpy (ΔH) were calculated using the software provided by Netzsch. The degree of crystallinity or recrystallization index (RI) was determined by the equation:| RI (%) = [ΔHNLC (J g−1)/ΔHbulk material (J g−1) × concentration lipid phase (%)] × 100 |
2.7. UV-vis analysis
The electronic spectra were recorded between 200 and 2000 nm on lyophilized free- and Ile loaded – lipid nanocarriers, using a Jasco double – beam V670 Spectrophotometer (diffuse reflectance analysis performed with the device ILN – 725 endowed with an integrating sphere).2.8. Determination of entrapment efficiency
Hederacoside C entrapment efficiency (EE) in NLC formulations was determined by HPLC. A standard solution of 0.1 g L−1 Hederacoside C has been used in the HPLC determination. For quantitative determination of Hederacoside C unloaded into NLC, 0.05 g lyophilized NLCs-Rbo/Pso/Rso-Ile and 1 mL water were mixed in a centrifugal tube and then centrifuged at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 155 RCF for 25 min (Sigma 2K15, Germany). The collected supernatant containing the unloaded-Ile was analysed using a Jasco 2000 liquid chromatograph equipped with a Nucleosil C18 column (5 μm, 25 × 0.4 mm) and a UV detector at λmax = 205 nm. The retention time of Hederacoside C was 12.6 min. The mobile phase was composed by ACN
155 RCF for 25 min (Sigma 2K15, Germany). The collected supernatant containing the unloaded-Ile was analysed using a Jasco 2000 liquid chromatograph equipped with a Nucleosil C18 column (5 μm, 25 × 0.4 mm) and a UV detector at λmax = 205 nm. The retention time of Hederacoside C was 12.6 min. The mobile phase was composed by ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H3PO4 = 70
H3PO4 = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and the flow rate was 1 mL min−1. The percent of loaded Hederacoside C has been calculated from the relation:
0.5 and the flow rate was 1 mL min−1. The percent of loaded Hederacoside C has been calculated from the relation:where the theoretic concentration of Hederacoside C was calculated starting from the content of Hed C in Ivy leaves extract, 14.65% Hed C and considering the initial Ile loading into NLCs (e.g. 1 g Ile for 100 g of aqueous NLCs dispersion).
2.9. Antioxidant activity
The in vitro antioxidant activity of the lipid nanocarriers loaded with ivy leaves extract and of free-nanocarriers was determined by chemiluminescence method using a Chemiluminometer Turner Design TD 20/20, USA. Luminol was used as a light amplifying substance in the presence of reactive oxygen species – hydrogen peroxide. H2O2 in Tris–HCl buffer solution (pH 8.6) was used as a generator system of free radicals. Dimethyl sulfoxide solutions were prepared with concentrations of 1 g L−1 lyophilized nanocarriers. The percentage of free radical scavenged by co-loaded NLCs was compared with the same concentration of free nanocarriers and was calculated by using the relation:where I0 and Is are the chemiluminescence maximum for standard and for sample at t = 5 s.
2.10. Cell cultures and treatments
All functional biological experiments were performed on cultures of murine fibroblast L929 and melanoma B16 cell lines. L929 cell line was purchased from the European Collection of Authenticated Cell Cultures (ECACC) through Sigma-Aldrich (St. Louis, Mo, USA); it is an adherent cell line, one of the first subclones of parental strain L established in continuous culture from normal subcutaneous areolar and adipose tissue of a 100 day old male C3H/an mouse, with morphology of fibroblasts (ECACC, catalogue no. 85011425). B16 cell line was a kindly gift from Prof. Stefan N. Constantinescu (MEXP Unit and Cell Signaling Unit, de Duve Institute, Universite Catholique de Louvain; Signal Transduction and Molecular Hematology Laboratory, Ludwig Institute for Cancer Research, Brussels, Belgium); it is an adherent cell line derived from a melanoma from the skin of a C57BL/6 strain mouse, showing fibroblast-like characteristics which produces melanin.Both cell lines were cultured and maintained in culture flasks, using a culture medium of DMEM/F12 supplemented with 2 mM L-glutamine and 10% fetal bovine serum, FBS (Sigma Aldrich, St. Louis, Mo, USA), incubated at 37 °C in a 5% CO2 humidified atmosphere. Stock solutions of NLCs were made by dissolving 20 mg in 1 mL DMSO; from the stock solution were made dilutions in culture medium. Cells were grown for 24 hours to achieve a density of 50%. After 24 h, either cells from flasks were detached with a non-enzymatic solution of PBS/1 mM EDTA and further used in cytobility assays, or the culture medium was discarded, cells were treated with increasing concentrations of lipid nanocarriers for various periods of time, and then detached, washed twice in PBS and immediately used for apoptosis assay.
2.11. Cytotoxicity assay (MTS)
All assays were performed in triplicate in 96-well microtiter plates with flat bottom (Falcon), using CellTiter 96 Aqueous One Solution Cell proliferation Assay (Promega), an MTS colorimetric assay. The method is based on the ability of metabolically active cells to reduce MTS, a yellow tetrazolium salt to the colored formazan that is soluble in the culture medium. Briefly, 15 × 103 cells per well were cultured in 100 μL for 24 h, culture supernatants discarded, then cells were treated for 24–72 h with increasing concentrations of NLCs. After the end of incubation time, 20 μL reagent containing (a) MTS, [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt], and (b) PES (phenazine ethosulfate) were added in each well. PES has a high chemical stability, that allows it to be combined with MTS to form a stable solution. After adding the colouring solution, plates were incubated 4 h at 37 °C, with mild agitation every 15 min. The colour developed during incubation was spectrophotometrically quantified at λ = 492 nm.2.12. Real-time cell analysis (RTCA)
The experiments were performed using xCELLigence technology and RTCA-DP analyzer that allows cell-based in vitro assays for the assessment of cell viability and cytotoxicity. Each well in E-plate incorporates a collection of sensor electrodes that allow monitoring and testing the cells in the well. The impedance measurement provides quantitative information about the biological status of the cells, including cell number, viability and morphology. Controlled by the RTCA software, RTCA DP analyzer can automatically select wells for the measurement of cell impedance and continuously transfers the data to the computer. Changes in a cell status led to a change in cell index (CI), which is a quantitative measure of cell number present in a well. One to three 16 wells E-plates could be used simultaneously or separately on RTCA-DP analyzer. Briefly, 15 × 103 cells per well were seeded in E-plates 16 cell (ACEA Biosciences) and cultured in 100 μL DMEM/F12 medium added with 2 mM L-glutamine and 10% FBS. Growth curves started to be automatically recorded on the xCELLigence System in real time. After 24 h scalar concentrations of NLCs were added to the E-plates 16, cells were incubated and impedance was measured every 15 min. Electronic sensors measure cell impedance via electrodes located at the bottom of each well, and the small changes in impedance are continuously measured by RTCA Instruments, and spatially integrated and expressed over time by the RTCA software 1.1 as Cell Index (CI). Plotted CI values were normalized to the last time point before the addition of NLCs.2.13. Apoptosis analysis by flow-cytometry
The apoptosis assay was carried out using the Annexin V-FITC Apoptosis Detection Kit and the manufacturer's protocol from Becton Dickinson (BD) Biosciences. Apoptotic events were evaluated by double staining of cells with Annexin V-FITC/PI, using the unmarked cells as negative control of the test. Binding Annexin V allows identification of the early and late stages of apoptosis, the process preceding necrotic processes. Staining with Annexin V in combination with a dye to assess the viability, such as propidium iodide (PI), is used order to assess the viability and discern between apoptotic and necrotic cells. Briefly, 105 cells per tube were resuspended in 100 μL binding buffer, added with 5 μL Annexin V/FITC and 5 μL PI solution, then incubated for 15 min at room temperature and dark conditions. Then 400 μL binding buffer were added and percentages of apoptotic events were measured by using data acquisition by flow-cytometry using a FAC Scan cytometer (Becton Dickinson, Immunocytometry System, Mountain View, CA). Evaluation of apoptotic events was performed using WinMDI 2.9 software.3. Results and discussion
3.1. The impact of surfactants and vegetable oils on the size and stability of NLC
Generally, the type and concentration of surfactant have a direct correlation with the particle size, encapsulation efficiency and stability of any colloidal carrier system. Given the hydrophilic nature of ivy leaves extract (Ile), comparison studies were firstly conducted in order to select the proper surfactant mixture for an efficient entrapment of bioactive compounds. Thus, two types of surfactants mixtures were evaluated for the preparation of free and Ile-loaded lipid nanocarriers, one predominantly nonionic o/w emulsifiers (composed by Tween 20, phosphatidylcholine and Poloxamer 407) and one ionic emulsifiers (sodium cholate, Tween 20 and Poloxamer 407), in association with lipid mixture composed by vegetable oil–Gso/Pso or Rbo, cetyl palmitate and glycerol monostearate. Fig. 2 shows that NLCs obtained with a constant amount of 3% vegetable oil and variable Ile concentrations (0.5 and 1%) had average diameters ranging from 100 and 140 nm. Although the selected vegetable oils have different content of polyunsaturated fatty acids (e.g. high amounts in ω-6 and ω-3 in case of Rso; ω-9 for Rgo and ω-5 for Pso), the position and the number of double bonds did not show a significant influence on the size distribution of lipid nanocarriers (Fig. 2). Higher average diameters were observed for NLCs prepared with pomegranate seed oil. Regarding the lipid particle size distribution in aqueous medium, reflected by the polydispersity index values, in most cases it was observed that PdI was less than 0.22, which highlights the existence of a narrow lipid population.Interesting results have been observed at encapsulation of plant extract. Generally, a decrease in the average diameters was detected after encapsulation of ivy leaf extract that has as main component a voluminous pentacyclic saponin, Hederacoside C (Fig. 1). A possible explanation may be given by the reorganization of the surfactants shell after capturing the plant extract, which led to the contraction of the lipid core and, thus, reducing the size of the lipid nanocarriers.
Referring to the influence of the surfactants mixture, all the selected vegetable oils have led to smaller average sizes for the lipid nanocarriers synthesized with a mixture of nonionic surfactants, e.g. 108.3 ± 0.289 nm with a PdI of 0.135 for NLC 9 (Rso) and 110.8 ± 1.002 nm with a PdI of 0.164 for NLC 23 (Rbo) which underlines a better coating capacity of the o/w emulsifiers on lipid core, aspect which is confirmed by the more electronegative values of the electrokinetic potential for the NLC formulations prepared with nonionic surfactant mixture (Table 1).
To confirm the previous size measurements evaluated by DLS analysis and to get more information about their morphology, microscopy analysis was performed on two representative nanocarriers prepared with Rso and Rbo. The results have highlighted the formation of homogeneous lipid nanocarriers with a well-defined spherical shape and size of ∼100 nm for NLCs prepared with raspberry seed oil and smaller than 150 nm for NLCs prepared with pomegranate seed oil (Fig. 3).
 | ||
| Fig. 3 TEM images of lipid nanocarriers prepared with Rso (a) and Rbo (b) loaded with ivy leaves extract. | ||
The development of a net charge on the particle surface affects the physical stability of the colloidal systems. In general, particles can be considered stably dispersed when the absolute value of the zeta potential is above 30 mV due to the electric repulsion among the particles. The investigated lipid systems manifested an appropriate stability, the zeta potential values ranging from −34.7 and −48.5 mV (Fig. 4), which indicates a reduced occurrence of the aggregation phenomena of lipid nanocarriers dispersed in aqueous media. Overall, the lipid nanocarriers prepared with a mixture of predominantly non-ionic surfactants showed values more electronegative than those prepared with a mixture of predominantly ionic surfactants (Fig. 4). The more electronegative zeta potential value determined for NLC 1 as compared to NLC 2 may be likely linked with different spatial arrangement of the complimentary ionic groups, i.e. cationic ammonium groups, anionic phosphate and carboxyl groups, in the outer shell of NLC.
 | ||
| Fig. 4 The effect of the vegetable oil on the physical stability of lipid nanocarriers loaded with different amounts of ivy leaves extract. | ||
Some variations have been seen for free- and Ile loaded-nanocarriers. For instance, a significant change of ξ has been observed on the NLC synthesized with Pso; in this case the potential values increased after Ile encapsulation (−48 mV for free-NLC to −39 mV for Ile loaded – nanocarriers prepared with predominantly non-ionic surfactant mixture). An opposite behavior occurs when using Rbo for preparation of vegetable nanocarriers; the ξ values for predominantly ionic surfactant mixture and loaded with Ile were more electronegative (−37 mV) than those encountered for free-lipid nanocarriers (−34 mV). The predominant electric charge of the double layer can be associated with individual and/or cumulative effects of Hederacoside C distribution and of the stabilization type offered by nonionic versus ionic surfactant. The hydrophilic extract will mostly prefer to accumulate in the outer shell of surfactants. This location of Ile modifies the repartition of surface charge due to the large number of OH groups from Hederacoside C.
3.2. Structural changes of lipid core after ivy leaves extract encapsulation into lipid nanocarriers
The evaluation of the polymorphic forms and crystallization behavior of the lipid core and the Ile encapsulation effect in different nanocarriers systems was achieved by differential scanning calorimetry (Fig. 5). The results of DSC analysis revealed two distinct endothermic peaks with corresponding polymorphic modifications and melting points in the range of 46 to 50 °C and of 52 to 55 °C, respectively (Fig. 5 and Table 2). The first peak with lower melting point could be attributed to the unsaturated fatty acids from the vegetable oils and the presence of α-polymorphic form, whereas the second peak specific to the solid lipids was attributed to the stable β-polymorphic form.| Samplea | Enthalpy (J g−1) | Melting point (°C) | Crystallinity index (%) | |
|---|---|---|---|---|
| a Bulk 1 ÷ 3 = physic mixture of lipids (MSG, CP and Rso/Pso/Rbo). | ||||
| Bulk 1 | 163.9 | 52.2 | 58.4 | 100 |
| NLC 1-Rso-Ile 2 | 130.5 | 47.3 | 52.1 | 79.6 |
| NLC 2-Rso-Ile 2 | 135.0 | 46.5 | 55.1 | 82.4 |
| NLC 1-Rso | 134.7 free | 47.2 | 52.7 | 82.2 |
| NLC 2-Rso | 137.4 free | 47.5 | 54.5 | 83.8 |
| Bulk 2 | 172.9 | 48.8 | 47.1; 61.9 | 100 |
| NLC 1-Pso-Ile 2 | 136.5 | 48.9 | 53.5 | 78.9 |
| NLC 2-Pso-Ile 2 | 122.8 | 46.5 | 55.2 | 71.0 |
| NLC 1-Pso | 143.4 free | 48.8 | 53.7 | 82.9 |
| NLC 2-Pso | 131.1 free | 47.5 | 55.4 | 75.8 |
| Bulk 3 | 161.3 | 49.8 | 57; 61.7 | 100 |
| NLC 1-Rbo-Ile 2 | 137.6 | 50.1 | 54.9 | 85.3 |
| NLC 2-Rbo-Ile 2 | 130.3 | 46.3 | 55.2 | 80.8 |
| NLC 1-Rbo | 142.0 free | 49.3 | 54 | 88.0 |
| NLC 2-Rbo | 133.6 free | 46.4 | 55 | 82.8 |
Endothermic peaks were very wide, suggesting the existence of highly disordered lipid cores which can be explained by the high content of polyunsaturated fatty acids. Most nanocarriers prepared with selected vegetable oils show slight changes of the lipid network before and after encapsulation of ivy leaves extract, reported for ΔH, melting point and crystallinity index (Fig. 7 and Table 2). For instance, a reduction of the crystallinity index from 100%, which is the conventionally considered value for the physical lipid mixtures, to 82.9% for free-lipid nanocarriers prepared with Pso (NLC 1-Pso) and to 78.9% for Ile loaded-NLC (NLC 1-Pso-Ile 2) is due to the partial formation of lower energy lipid modifications, resulting in a more disordered crystalline lattice of the lipid network (Table 2).
By the encapsulation of the hydrophilic Ile mixture, a slight deceleration of the endothermic peak and melting point values for lyophilized NLCs containing 7.4% Ile (Fig. 5A–C) was observed. However, the presence of Ile caused a slight reduction of the melting enthalpy, e.g. from 133.6 to 130.3 J g−1 for NLC 2-Rbo and NLC 2-Rbo-Ile 2, suggesting a lower level of organization of the lipid lattice in the presence of Ile. The crystallinity index value also decreased slowly when Ile was incorporated, indicating that Ile induces disorder in the crystal structure of the nanocarriers. The lowest crystalline degree was obtained for NLC based on pomegranate seed oil and prepared with ionic surfactant (e.g. 75.8% for NLC 2-Pso and 71% for NLC 2-Pso-Ile 2).
These small variations in DSC parameters indicate a minor disturbance of the lipid network formed by the solid lipids with the vegetable oil. The results can be correlated with the loading capacity of the lipid core versus the outer shell of surfactants. In other words, the encapsulation of the hydrophilic Ile led to its predominant repartition in the surfactants coating, but the presence of significant amounts of Ile in the lipid core cannot be excluded. The ivy leaves extract is most likely is retained in the surfactant coating through weak interactions, e.g. hydrogen bonds that occur due to the large number of hydroxyl groups, both found in the vegetable extract and surfactants used for the synthesis of lipid nanocarriers.
3.3. Quantifying the Ile encapsulation by evaluating the spectral and chromatographic characteristics
A first assessment of the encapsulation effect of ivy extract into lipid nanocarriers was performed by spectral characterization. Given that ivy extract and vegetable oils are complex mixtures, the UV-vis absorption bands are in agreement with their structure and composition, e.g. in most nanocarriers an overlapping of absorption peaks of functional groups was detected. The primary feature of UV-vis spectra of free NLC, Ile loaded – NLC and native Ile (Fig. 6A) has shown a wide range of absorption from 200 to 450 nm. The presence of a weak shoulder between 200 and 320 nm can be attributed to electronic transitions n–π*, π–π* of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C found in surfactants, lipids and vegetable actives (i.e. saponins, flavones, carotenoids, polyphenols, chlorogenic acids etc.). For instance, the conjugation system of punicic acid (ω-5, predominantly in Pso), linoleic acid (ω-6, predominantly in Rso) are highlighted in the region of 260–280 nm. The wide region from 400–490 nm absorption bands encountered only in the Ile and Ile-NLC indicates the presence of significant amounts of carotenoids that are responsible for yellow-brownish color of the ivy extract. One of the bands of Hederacoside C also specific for double bonds (206 nm) is very difficult to be observed, being situated at the broad absorption from 200–500 nm.
C found in surfactants, lipids and vegetable actives (i.e. saponins, flavones, carotenoids, polyphenols, chlorogenic acids etc.). For instance, the conjugation system of punicic acid (ω-5, predominantly in Pso), linoleic acid (ω-6, predominantly in Rso) are highlighted in the region of 260–280 nm. The wide region from 400–490 nm absorption bands encountered only in the Ile and Ile-NLC indicates the presence of significant amounts of carotenoids that are responsible for yellow-brownish color of the ivy extract. One of the bands of Hederacoside C also specific for double bonds (206 nm) is very difficult to be observed, being situated at the broad absorption from 200–500 nm.
A distinct difference in the absorption characteristics is observed in the vis domain of free- and Ile loaded-NLC, by means the presence of a weak absorption band located at 670 nm which appears in the ivy extract and Ile-NLC spectra, but it is absent in the unloaded-lipid nanocarriers spectrum. According to literature data, chlorophylls dye from Ile are responsible for the absorption maximum at around 670 nm.38 Identifying chlorophyll after capturing the ivy extract inside the lipid nanocarriers is an indicative of protecting the active principle by the solid lipid matrix as well as a benefit in terms of chlorophyll use as an encapsulation marker.
Another noteworthy encapsulation effect arises from the changes of the optical density as the ivy extract was entrapped into NLCs. By far, the clear evidence of ivy extract presence inside the nanocarriers was obtained by determination of one pharmaceutically active constituent – Hederacoside C, by HPLC analysis. The quantitative determination of Hederacoside C in selected Ile-NLCs has revealed no difference of entrapment efficiency inside the lipid nanocarriers prepared with rice bran, pomegranate or raspberry seed oils (Fig. 6B). For the lyophilized nanocarriers that contain 22.2% raspberry oil/rice/pomegranate oil and 7.4% ivy leaf extract an efficiency of 82% to entrap Hederacoside C was identified. The high entrapment efficiency of Hederacoside C proves the preservation of the ivy extract structural integrity after its encapsulation into lipid nanocarriers.
3.4. The effectiveness of ivy leaves extract loaded-NLC to scavenge free oxygen radicals
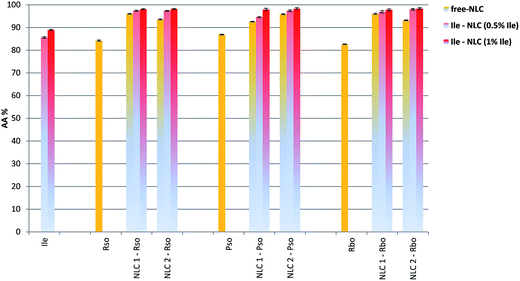
Rapid and effective in vitro evaluation of the antioxidant activity of Ile-NLC was assessed by chemiluminescence technique. For this purpose different dimethyl sulfoxide solutions of vegetable oils (Pso, Rso and Rbo), ivy leaves extract, free- and Ile loaded-NLCs were subjected to oxygen free radicals generated in situ by using the luminol and hydrogen peroxide system.39 A comparative analysis of the antioxidant results has revealed no significant differences by varying the type of vegetable oil and the main surfactant (Fig. 7). The obvious difference in the degree of capturing free radicals was determined by encapsulating ivy leaves extract into NLCs compared with free-NLCs. Generally, the antioxidant activity was less pronounced when the ionic surfactant was predominantly used, i.e. 97.18% ± 0.48 (for NLC based on Rso); 98.29% ± 0.39 (for NLCs prepared with Pso); 98.3% ± 0.5 (NLCs based on Rbo).A good antioxidant capacity was also observed for the native vegetable oils (e.g. between 83 and 87%), but their coupling with ivy extract in conjunction with nanoscale feature, obviously led to an enhancement of this property. For almost all Ile-loaded nanocarriers solutions (0.15 g L−1 NLCs), a capacity of 94 and 98% to capture free radicals was identified. The functionality of Ile-NLCs which shows the ability to capture or deactivate 98% of free radicals, could be mainly driven by the Ile molecular structure consisting of hydroxyl groups, as well as by the omega-3, -5, -6 and -9 advantages from vegetable oils and not least by capturing both vegetable actives in the same nanocarrier system.
3.5. Observational study on the safety and anti-tumor performance of Ile-NLCs based on vegetable oils against normal and tumor cell lines
Since phytochemicals comprise complex active compounds, some constituents can manifest cytotoxic action against various cell lines or may contribute to the efficacy of lipid nanocarriers based on vegetable oils. In this respect the first issue addressed is the safety of the developed lipid nanocarriers. The results obtained by the investigation of proliferative vs. cytotoxic action of NLCs on two cell lines, normal L929 cells and tumor B16 cells, are varied depending on the lipid nanocarriers concentration, the treatment time, and the cell line subjected to the treatment (Fig. 8). | ||
| Fig. 8 Cytotoxicity induced by Ile-loaded NLC treatment (48 h and 72 h) against normal L929 and tumor B16 cell lines. Data were expressed as mean ± SDV (n = 3). | ||
In general, Ile-loaded NLCs based on rice brain oil exhibit a more pronounced proliferative effect on L929 cells (at 48 h treatment), compared with Ile-NLCs synthesized with Rso or Pso (Fig. 8A). However, the cell viability of L929 cells after incubation for 72 h with nanocarrier systems loaded with herbal extract, was maintained at values higher than 92% for all the tested concentrations (from 3 to 200 μg mL−1 NLCs), suggesting a lack of toxicity of the developed NLCs based on Rso/Pso or Rbo. An effective action of developed NLCs against tumor B16 cell line was detected for Ile-NLC synthesized with Rbo (Fig. 8B). It caused obvious cytotoxicity against tumor B16 cell line, but the effect was stronger when treatments were prolonged till 72 h. The highest effect was observed for Ile-NLC synthesized with Rbo since the viability decreased up to 64% when cell were treated with 200 μg mL−1 for 72 h (Fig. 8B); moreover, both 50 and 200 μg mL−1 treatments with Ile-NLC synthesized with Rbo induced the highest apoptotic changes in B16 tumor cells (Fig. 10B).
In order to confirm the proliferative vs. cytotoxicity capacity of the lipid nanocarriers on tumor B16 cells, the RTCA assay was used. RTCA allows the measuring in real time of the cell index40 and offers the possibility to calculate the ratio viability/cytotoxicity of 50% (IC50) for each concentration/treatment and in any point of the time-dependent curves (Fig. 9). Test results obtained by RTCA on B16 melanoma cells add the data obtained by MTS assay. Although different cytotoxic agents have different kinetics, agents with a similar mode of action have similar time-dependent cell response profiles. RTCA analyses showed that increasing periods of time for all treatments induced a higher cytotoxic effect. All Ile-NLC compounds tested demonstrated reduced viability at the end of the study; however, the kinetic response seems to suggest differential time-dependent cytotoxic effects. Ile-NLC-mediated cytotoxicity became apparent 24 hours after compound addition for 100, 200 and 400 μg mL−1 treatments. It reached its maximal effect at 86, 80 and 76 h after treatment with Ile-NLC prepared with 400 μg mL−1 of Pso, Rso, and Rbo, respectively (Fig. 9).
 | ||
| Fig. 9 Cytotoxic vs. proliferative action induced by NLC against tumor B16 cell line, by xCELLigence real time cell analysis. | ||
Ile-NLCs based on Rbo produced a stronger inhibitory effect on B16 cell proliferation than Ile-NLCs prepared with Pso or Rso. According to RTCA assay, the cytotoxicity of 50% can be achieved in B16 cell line after treatment for 60 h with a higher concentration of NLC 1-Pso-Ile 2 (90 μg mL−1) compared to NLC 1-Rso-Ile 2 (24 μg mL−1), respectively NLC 1-Rbo-Ile 2 (11.3 μg mL−1) (Table 3).
| Type of NLC | 36 h | 42 h | 48 h | 60 h | 72 h |
|---|---|---|---|---|---|
| IC50 (μg mL−1) | IC50 (μg mL−1) | IC50 (μg mL−1) | IC50 (μg mL−1) | IC50 (μg mL−1) | |
| NLC 1-Pso-Ile 2 | 413 | 74 | 61 | 24 | 21 |
| NLC 1-Rso-Ile 2 | 277 | 158 | 129 | 90 | 38 |
| NLC 1-Rbo-Ile 2 | 475 | 151 | 57 | 11.3 | 26 |
To further elucidate the biological effects of Ile-NLC compounds, we investigated the modulation of apoptosis induced by Ile-NLC via flow cytometry. Fig. 10 shows the histograms of total apoptosis for normal L929 and tumor B16 cell lines treated with two concentrations of Ile-NLC (50 and 200 μg mL−1) for 48 h. The flow cytometry data reveals that there is an obvious difference in the amount of apoptotic events induced by the three Ile-NLC demonstrated by Annexin V labeled cells. All Ile-NLCs under study caused apoptosis induction in tumor B16 cells that increased with the dose of treatment. The percentages of apoptotic events determined by a treatment with 200 μg mL−1 NLC 1-Rbo-Ile 2 was of 37.83%, while for NLC 1-Rso-Ile 2 and NLC 1-Pso-Ile 2 were 34.51% and 23.76%, respectively (Fig. 10B and Table 4). Regarding the effect of developed ivy leaves extract loaded-NLC based on Rso/Pso/Rbo on the normal L929 cells, the apoptosis induction is influenced by a dose-dependent manner (Fig. 10A). By comparing the role of NLCs in apoptosis induction in B16 cell lines, the data suggest that NLC 1-Rbo-Ile 2 showed a more significant apoptosis induction in the tumor cells compared to NLC 1-Pso-Ile 2 or NLC 1-Rso-Ile 2 for both concentrations used (Fig. 10B and Table 4). Regarding the effect of developed ivy leaves extract loaded-NLC based on Rso/Pso/Rbo on the normal L929 cells, the apoptosis induction is influenced in a dose-dependent manner (Fig. 10A), but levels are much lower than those induced in B16 cells (Table 4).
| Treatment | Apoptosis (%) | ||
|---|---|---|---|
| Type of NLC | NLC conc. (μg mL−1) | B16 cell line | L929 cell line |
| Untreated | — | 4.53 | 2.3 |
| NLC 1-Pso-Ile 2 | 50 | 15.69 | 6.28 |
| 200 | 23.76 | 13.23 | |
| NLC 1-Rso-Ile 2 | 50 | 16.69 | 6.86 |
| 200 | 34.51 | 19.19 | |
| NLC 1-Rbo-Ile 2 | 50 | 27.28 | 8.27 |
| 200 | 37.83 | 13.88 | |
4. Conclusions
The combined effect of hydrophilic and lipophilic active compounds from various medicinal herbs and lipid nanoparticles on designing safe and efficient nanoscaled phytochemical formulations was investigated. The Ile-loaded NLCs had average diameters of 108–142 nm and polydispersity index values less than 0.22. An increase of particle size for Ile-loaded nanocarriers was observed, though it was more significant for those systems prepared with pomegranate seed oil. The change of surfactant mixture did not significantly affect the zeta potential of NLCs (e.g. –37 mV to −46 mV).According to scanning calorimetry study, the lipid nanocarriers showed amorphous characteristics, with a slight deceleration of the endothermic peak and melting points for Ile loaded – nanocarriers as compared to empty NLCs. The lyophilized nanocarriers containing 22.2% vegetable oils and 7.4% ivy leaves extract assured an efficiency of 82% to entrap the Hederacoside C from the ivy extract, thus confirming the preservation of the extract structural integrity after its encapsulation.
The high antioxidant activity encountered for all Ile-NLCs (by scavenging between 94 and 98% free oxygen radicals) can be explained by the presence of ω-3, -5, -6 and -9 from vegetable oils, coupled with the ivy extract and not least by capturing both vegetable actives in the same nanocarrier system.
Lipid nanocarriers loaded with ivy leaves extract caused a significant inhibition of B16 cell proliferation in a concentration- and time-dependent manner. A treatment with 200 μg mL−1 Ile-NLCs based on Rbo/Pso/Rso for 72 h did not affect the viability of normal L929 cell lines, the viability being maintained above 92%. Instead, Ile-NLC based on Rbo has a more pronounced effect in inhibiting proliferation of B16 cells (e.g. 64% viability for 72 h treatment) as compared to Ile loaded-NLCs synthesized with Rso and Pso, and induced obvious apoptotic changes in the B16 tumor cells (e.g. 38% apoptosis).
Remarkably, the antioxidant property, biocompatibility and cytotoxicity effect of these Ile-loaded lipid nanocarriers facilitate their application as potent delivery system for phytochemicals with effective action in cancer therapy.
Acknowledgements
The work was supported by a grand of the Ministry National Education, CNCS – UEFISCDI, project number PCE_PN-II-ID-PCE-2012-4-0111.References
- Y. Gan, X. Tong, L. Li, S. Cao, X. Yin, C. Gao, C. Herath, W. Li, Z. Jin, Y. Chen and Z. Lu, Int. J. Cardiol., 2015, 183, 129–137 CrossRef PubMed.
- H.-M. Ju, K.-W. Yu, S.-D. Cho, S. H. Cheong and K. H. Kwo, Compl. Ther. Med., 2016, 24, 47–54 CrossRef PubMed.
- W. Li, S. Yi, Z. Wang, S. Chen, S. Xin, J. Xie and C. Zhao, Int. J. Pharm., 2011, 4209, 161–171 CrossRef PubMed.
- J. R. Nakkala, R. Mata, A. K. Gupta and S. R. Sadras, Eur. J. Med. Chem., 2014, 85, 784–794 CrossRef CAS PubMed.
- A. Bryła, G. Lewandowicz and W. Juzwa, J. Food Eng., 2015, 167, 189–195 CrossRef.
- A. F. Esfanjani, S. M. Jafari, E. Assadpoor and A. Mohammadi, J. Food Eng., 2015, 165, 149–155 CrossRef CAS.
- A. Mohammadi, S. M. Jafari, A. F. Esfanjani and S. Akhavan, Food Chem., 2015, 190, 513–519 CrossRef PubMed.
- J. Varshosaz, F. Hassanzadeh, H. Sadeghi and S. Andalib, Eur. J. Med. Chem., 2012, 54, 429–438 CrossRef CAS PubMed.
- S. V. Mussi and V. P. Torchilin, J. Mater. Chem. B, 2013, 1, 5201–5209 RSC.
- Y. Liu, L. Wang, Y. Zhao, M. He, X. Zhang, M. Niu and N. Feng, Int. J. Pharm., 2014, 476, 169–177 CrossRef CAS PubMed.
- G. Fang, B. Tang, Y. Chao, Y. Zhang, H. Xu and X. Tang, RSC Adv., 2015, 5, 96437–96447 RSC.
- S. K. Singh, P. Dadhania, P. R. Vuddanda, A. Jain, S. Velaga and S. Singh, RSC Adv., 2016, 6, 2032–2045 RSC.
- S. Doktorovova, E. B. Souto and A. M. Silva, Eur. J. Pharm. Biopharm., 2014, 87, 1–18 CrossRef CAS PubMed.
- J. Zhu, P. Zhuang, L. Luan, Q. Sun and F. Cao, J. Funct. Foods, 2015, 19, 902–912 CrossRef CAS.
- W. Li, T. Zhang, Y. Ye, X. Zhang and B. Wu, Int. J. Pharm., 2015, 495, 948–955 CrossRef CAS PubMed.
- G. Niculae, I. Lacatusu, N. Badea, R. Stan, B. S. Vasile and A. Meghea, Photochem. Photobiol. Sci., 2014, 13, 703–716 CAS.
- I. Lacatusu, N. Badea, G. Badea, O. Oprea, M. A. Mihaila, D. A. Kaya, R. Stan and A. Meghea, Mater. Sci. Eng., C, 2015, 56, 88–94 CrossRef CAS PubMed.
- L. Arana, C. Salado, S. Vega, O. Aizpurua-Olaizola, I. de la Arada, T. Suarez, A. Usobiaga, J. L. R. Arrondo, A. Alonso, F. M. Goñi and I. Alkorta, Colloids Surf., B, 2015, 135(2015), 18–26 CrossRef CAS PubMed.
- D. A. Campos, A. R. Madureira, B. Sarmento, A. M. Gomes and M. M. Pintado, Food Res. Int., 2015, 78, 131–140 CrossRef CAS.
- J. Wang, H. Wang, R. Zhu, Q. Liu, J. Fei and S. Wang, Biomaterials, 2015, 53, 475–483 CrossRef CAS PubMed.
- D. F. Cortés-Rojas, C. R. F. Souza and W. P. Oliveira, J. Food Eng., 2014, 127, 34–42 CrossRef.
- E. Mitrea, I. Lacatusu, N. Badea, C. Ott, O. Oprea and A. Meghea, J. Nanosci. Nanotechnol., 2015, 15, 4080–4089 CrossRef CAS PubMed.
- D. Istrati, I. Lacatusu, N. Bordei, G. Badea, O. Oprea, L. M. Stefan, R. Stan, N. Badea and A. Meghea, Mater. Sci. Eng., C, 2016, C64, 249–259 CrossRef PubMed.
- S. Fazio, J. Pouso, D. Dolinsky, A. Fernandez, M. Hernandez, G. Clavier and M. Hecker, Phytomedicine, 2009, 16, 17–24 CrossRef CAS PubMed.
- C. Greunke, A. Hage-Hülsmann, T. Sorkalla, N. Keksel, F. Häberlein and H. Häberlein, Pulm. Pharmacol. Ther., 2015, 31, 92–98 CrossRef CAS PubMed.
- U. Cwientzek, B. Ottillinger and P. Arenberger, Phytomedicine, 2011, 18, 1105–1109 CrossRef PubMed.
- M. Yu, Y. J. Shin, N. Kim, G. Yoo, S. J. Park and S. H. Kim, J. Chromatogr. Sci., 2014, 53, 478–483 Search PubMed.
- M. Pieszka, B. Tombarkiewicz, A. Roman, W. Migdał and J. Niedziółka, Environ. Toxicol. Pharmacol., 2013, 36, 1055–1062 CrossRef CAS PubMed.
- C. S. Bowen-Forbes, Y. Zhang and M. G. Nair, J. Food Compos. Anal., 2010, 23, 554–560 CrossRef CAS.
- V. Verardo, P. Garcia-Salas, E. Baldi, A. Segura-Carretero, A. Fernandez-Gutierrez and M. F. Caboni, Food Res. Int., 2014, 65, 445–452 CrossRef CAS.
- M. Mizrahi, Y. Friedman-Levi, L. Larush, K. Frid, O. Binyamin, D. Dori, N. Fainstein, H. Ovadia, T. Ben-Hur, S. Magdassi and R. Gabizon, Nanomedicine: Nanotechnology, Biology and Medicine, 2014, 10, 1353–1363 CrossRef CAS PubMed.
- M. Spilmont, L. Léotoing, M. J. Davicco, P. Lebecque, S. Mercier, E. Miot-Noirault, P. Pilet, L. Rios, Y. Wittrant and V. Coxam, J. Nutr. Biochem., 2013, 24, 1840–1848 CrossRef CAS PubMed.
- N. Samad, Rice bran oil prevents neuroleptic-induced extrapyramidal symptoms in rats: possible antioxidant mechanisms, J. Food Drug Anal., 2015, 23, 370–375 CrossRef CAS.
- L. A. Rigo, C. Regina da Silva, S. Marchesan de Oliveira, T. Nunes Cabreira, C. Bona da Silva, J. Ferreira and R. C. R. Beck, Eur. J. Pharm. Biopharm., 2015, 93, 11–17 CrossRef CAS PubMed.
- A. Salar, S. Faghih and G. R. Pishdad, J. Clin. Lipidol., 2016, 10, 299–305 CrossRef PubMed.
- G. Niculae, N. Badea, A. Meghea, O. Oprea and I. Lacatusu, Photochem. Photobiol., 2013, 89, 1085–1094 CrossRef CAS PubMed.
- I. Lacatusu, G. Niculae, N. Badea, R. Stan, O. Oprea and A. Meghea, Chem. Eng. J., 2014, 246, 311–321 CrossRef CAS.
- R. F. Correia, M. I. Viseu and S. M. Andrade, Photochem. Photobiol. Sci., 2014, 13, 907–916 CAS.
- K. Papadopoulos, T. Triantis, E. Yannakopoulou, A. Nikokavoura and D. Dimotikali, Anal. Chim. Acta, 2003, 494, 41–47 CrossRef CAS.
- C. Knop, J. Putnik, M. Scheuermann and M. Schmitz, Cutting Edge Technologies: Cell Analysis, ed. B. Ziebolz, Heidelberg, 2010, pp. 4–13, 58–68, 137–141 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |