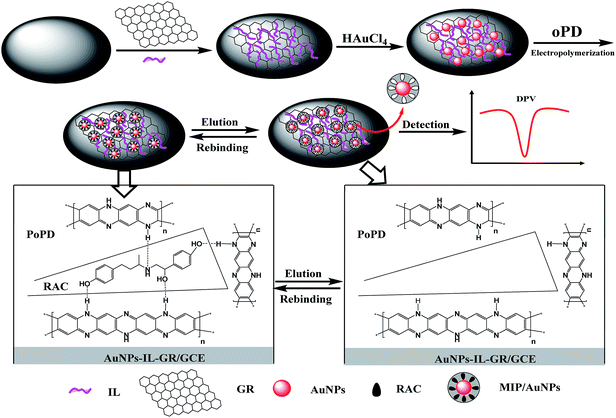
Electrochemical detection of ractopamine based on a molecularly imprinted poly-o-phenylenediamine/gold nanoparticle–ionic liquid–graphene film modified glass carbon electrode†
Tengfei Liab,
Ting Yaoac,
Chao Zhanga,
Guangyang Liua,
Yongxin She*a,
Maojun Jina,
Fen Jina,
Shanshan Wanga,
Hua Shaoa and
Jing Wang*a
aKey Laboratory for Agro-Products Quality and Food Safety, Institute of Quality Standards & Testing Technology for Agro-Products, Chinese Academy of Agricultural Sciences, Beijing 100081, China. E-mail: 0891syx@163.com; w_jing2001@126.com; Fax: +86-10-82106567; Tel: +86-10-82106513
bDepartment of Food Science, College of Agriculture, Hebei University of Engineering, Handan 056021, China
cBeijing Institute of Feed Control, Beijing 100020, China
First published on 5th July 2016
Abstract
An electrochemical sensor for sensitive detection of ractopamine (RAC) was fabricated by using molecularly imprinted polymer (MIP) incorporation with graphene (GR), ionic liquid (IL) and gold nanoparticle (AuNPs) nanocomposites. The AuNPs–IL–GR nanocomposite was utilized to improve the electrochemical response while MIP served as a recognition element. The MIPs were prepared by electropolymerization of o-phenylenediamine (oPD) on the AuNPs–IL–GR nanocomposite modified electrode. The resulting sensor was studied with respect to its response to hexacyanoferrate as a probe and characterized by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). Differential pulse voltammetry (DPV) was used to investigate the electrochemical behaviors of the proposed sensor in a phosphate buffer solution. Under optimal conditions, the peak current was linear to RAC concentration in the range from 10 μg L−1 to 5000 μg L−1, with a low detection limit of 0.46 μg L−1 (S/N = 3). The electrochemical MIP-sensor was applied to the determination of RAC in swine urine samples and satisfactory results were obtained.
1. Introduction
Ractopamine (RAC), a typical β-adrenergic agonist, was originally used as a bronchial dilating agent for the treatment of pulmonary disease and asthma.1 However, it is also illegally applied in the farming industry as a nutrient-repartitioning agent to improve muscle tissue growth in livestock.2,3 Because of the potential risk to consumers for adverse cardiovascular and central nervous system effects,4,5 RAC is not licensed for animal production in many countries. To ensure food safety, China and some European countries set strict regulations for β-adrenergic agonists as zero tolerances in animal foods.6,7 Therefore, the detection of RAC residues in animal tissues is quite important. Methods for RAC determination include high-performance liquid chromatography (HPLC),8 gas chromatography-mass spectrometry (GC-MS),9 ultra-performance liquid chromatography-tandem mass spectrometry (LC-MS),10 capillary electrophoresis (CE).11 These methods are sensitive and reliable, but in the meantime expensive for instrumentation, cumbersome for sample pretreatment and time-consuming for detection procedure. Consequently, it is urgent to develop easier, faster, and more sensitive methods for detecting RAC.Electrochemical sensors, with the advantages of sensitivity, good selectivity, speediness and potential for on-site monitoring are regarded as promising alternatives to conventional detectors. To date, various electrochemical sensors such as graphene oxide modified electrode, three-dimensional nanocomposite of reduced oxide graphene and Cu/Cu2O nanocrystal-based electrochemical biosensor, and gold nanoparticles/poly(dimethyldiallylammonium chloride)–graphene composites-based electrochemical aptasensor have been reported for the detection of RAC.12–14
It is found that the recognition element plays an important role in the electrochemical sensors. Chemically sensitive materials or natural biological molecules such as antibodies, aptamers, or enzymes are frequently used as recognition elements.15 However, these recognition elements often suffer from poor stability against high temperature, harsh chemical environments, or extreme pH values, making them less-desired. A new trend in analytical chemistry is to develop novel sensors based on molecularly imprinted polymers (MIPs). MIPs are reported with high specificity, and the reaction model between MIPs and target molecules are quite similar to that of the antibodies. Due to its superior stability, low cost and easy preparation,16 MIPs are widely used for separation,17,18 biosensing,19,20 and extraction.21,22 Various sensors based on MIPs have already been applied for rapid determination of β2-agonists in real samples.23–26 Moreover, from the preparation point of view, the rapidly developed methods make MIP potential functional materials for electrochemical sensors.27–30 For example, with in situ electropolymerization method, an insulating and ultrathin polymeric film can be easily grown adherent to a conducting electrode of any shape and size and with a thickness controlled by the amount of circulated charge.31 To increase the amount of effective imprinted sites on the sensor surface, the simplest method is to use a higher surface area electrode, through the assembly of nanomaterials at the surface of electrodes.
Graphene (GR), a two-dimensional material with single-atom thickness, is widely studied in electrochemical analysis for its large surface area, rich functional groups, and good conductivity.32,33 Gold nanoparticles (AuNPs) have been widely used as a signal amplification medium to increase sensitivity of sensors because of their unique chemical and physical features.23,34 Studies have shown that GR improves the sensitivity and signal responses of electrochemical sensors when combined with nanomaterials.35 For a MIP-based sensor, assembled AuNPs–GR can increase the sensor surface area, and thus increase the total number of imprinted sites formed in the polymer matrix. This in turn produces more sensitive assays.
Ionic liquid (IL) are low-melting-point salts, thus forming liquids that consist only of cations and anions. For the overall environmental impact and economics, IL have been proposed as “green” alternatives to conventional organic solvents in a range of applications, such as electrochemistry,36 analytical chemistry,37 chemical synthesis,38 and liquid–liquid extractions.39 Particularly, they are widely used in the fabrication of electrochemical sensors due to their non-volatility, good film-formation ability, high ionic conductivity and large electrochemical window.40
Herein, we report a surface molecular self-assembly strategy for molecular imprinting in electropolymerized o-phenylenediamine (oPD) membranes at the AuNPs–IL–GR-modified electrode for the electrochemical detection of RAC. The AuNPs–IL–GR nanocomposites are expected to act not only as substrates for MIP immobilization, but also as an electron transfer promoter or mediator. SEM was used for surface characterization. CV, DPV, and EIS were used as sensing techniques. The experimental parameter such as template to functional monomer ratio, pH value, and incubation time were optimized. In addition, this sensitive electrochemical MIP-sensor was successfully applied to the determination of RAC in real samples.
2. Experimental
2.1 Reagents and materials
RAC, clenbuterol (CL), and salbutamol (SAL) were obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany); o-phenylenediamine (oPD) was purchased from Fuchen Chemical Reagent Co., (Tianjin, China); 1-butylpyridinium hexafluorophosphate (BPPF6, >99% Lanzhou Greenchem ILS. LICP. CAS, China); HAuCl4·4H2O was purchased from Sinopharm Chemical Reagent Beijing Co., Ltd. (Beijing, China) and GR was from JCNANO (Nanjing, China). Potassium ferricyanide (K3[Fe(CN)6]) and other chemicals used in the experiments were purchased from Beijing Chemical Reagent Co., (Beijing, China) and at least of analytical grade. The buffers used in this study were as follows: phosphate buffer solution (PBS) (0.01 mol L−1), acetate buffer solution (ABS) (0.1 mol L−1, pH 5.2). Doubly deionized water (DDW, 18.2 MΩ cm−1) was purified using the Milli-Q reagent water system plus from Millipore.2.2 Instrumentation and operation parameters
All electrochemical experiments were performed using CHI 630E electrochemistry workstation (Shanghai CH Instruments, China) with a conventional three-electrode system, consisting of a bare or modified glass carbon electrode (GCE) as the working electrode, a saturated calomel electrode (SCE) as the reference electrode, and a platinum wire as the counter electrode. Transmission electron microscopy (TEM) images were taken on a JEOL JEM 2100F transmission electron microscope with an accelerating voltage of 200 kV. AFM measurements were made using the DI Multimode SPM from Veeco Systems and the images were obtained under the tapping mode. Scanning electron microscope (SEM) images were recorded with an S-4000 SEM (Hitachi, Japan). All electrochemical measurements were performed at room temperature.2.3 Preparation of AuNPs–IL–GR/GCE
A GCE (3 mm in diameter) was polished to a mirror-like finish with 0.3 μm and 0.05 μm alumina slurry, and were then successively in an ultrasonic cleaner with water and ethanol. Prior to the surface modification, the bare GCE was cyclic-potential scanned in the potential range −0.2 V to +0.6 V in 5.0 mmol L−1 K3[Fe(CN)6] solution containing 0.1 M KCl supporting electrolyte until a pair of well defined redox peaks was observed. To prepare GR/GCE, 10 μL of the GR–DMF suspension (0.5 mg mL−1) was dropped onto the surface, and then 10 μL of IL solution (5 μL BPPF6, 0.5 mL water, and 0.5 mL acetone, vortexed and sonicated before use) was dropped onto the GR/GCE.Moreover, gold nanoparticles (AuNPs) were electrochemically deposited onto the surface of IL–GR/GCE to obtain AuNPs–IL–GR/GCE. Before the deposition of AuNPs, IL–GR/GCE was active in 0.2 M H2SO4 by cyclic voltammetric scan between −0.8 V and +1.3 V for 20 cycles.41 Then the IL–GR/GCE was immersed into the 0.2 g L−1 HAuCl4 solution (with acetonitrile as a solvent and 5 mmol L−1 tetrabutyl ammonium perchlorate added to improve electrical conductivity) and treated via electropolymerization (with cyclic voltammetry for 10 cycles in the potential range −0.2 V to +1.0 V at a scan rate of 50 mV s−1) to electrodeposit the AuNPs onto its surface.
2.4 Preparation of the imprinted PoPD/AuNPs–IL–GR/GCE
PoPD modification was carried out by methods that were reported in the literatures.42,43 The pretreated AuNPs–IL–GR/GCE was immersed into a 10 mM oPD acetonitrile solution. After a reaction period of 24 h at room temperature, the electrode was taken out and rinsed with ethanol and water. Then, the oPD modified AuNPs–IL–GR/GCE was immersed into an electropolymerization solution containing 30 mmol L−1 oPD, 10 mmol L−1 RAC, and HAc–NaAc (pH 5.3) as the solvent. In this solution, the MIPs were produced via electropolymerization (with 10 CV cycles in the potential range −1.0 to +1.0 V and at a scan rate of 50 mV s−1). The electrode was taken out, rinsed with ethanol, and then dried under nitrogen flow at the room temperature. RAC molecules accordingly assembled onto the oPD modified AuNPs–IL–GR/GC electrode through hydrogen bond interactions between the residual amino groups (–NH2) of oPD and the nitrogen/oxygen atom of RAC. The NIP (non-molecular imprinted polymer) electrode was prepared in the same way as the control electrode, whereby the template molecule was not added during the electropolymerization stage.To remove the RAC template, both MIP and NIP electrodes were treated in a PBS (pH 7.0) solution by 5 CV cycles in the potential range 0 V to 2.0 V and at a scan rate of 100 mV s−1. The electrode was then rinsed with double distilled water and ethanol and dried with nitrogen for further use.
2.5 Electrochemical detection of RAC
Since the DPV technique was more sensitive than CV, serial dilutions of ractopamine standard solutions were applied to investigate its electrochemical methodology on MIP-sensor using DPV. The MIP/AuNPs–IL–GR/GCE was dipped into a standard solution containing the concentration of RAC ranging from 10 μg L−1 to 5.0 mg mL−1 for 600 s, washed with double-distilled water carefully to remove the possible adsorptive substances on the electrode surface, and then transferred to the electrochemical cell containing 5.0 mmol L−1 K3[Fe(CN)6] and 0.1 M KCl solution. Differential pulse voltammetry (DPV) was then performed in the potential range of −0.2 to +0.6 V (vs. SCE).3. Results and discussion
3.1 Fabrication principles of the electrochemical MIP-sensor
The fabrication process of the MIP/AuNPs–IL–GR composite-modified electrochemical MIP-sensor is shown in Scheme 1.Inspired by the efficient redox-activity, high electrical conductivity and loading ability of the AuNPs–IL–GR nanocomposite, an electrochemical MIP-sensor was fabricated by electropolymerization of oPD on the surface of AuNPs–IL–GR/GCE. The preparation was summarized as four steps: casting GR and IL on the surface of GCE; electrodeposition of AuNPs on the surface of IL–GR/GCE; electropolymerization of oPD on the surface of AuNPs–IL–GR/GCE; removal of the imprinting RAC molecules from the imprinted PoPD membranes. Following that the MIP/AuNPs–IL–GR/GCE was dipped into a standard solution containing the RAC. Considering that RAC is electro-inactive over the studied potential range, an electroactive substance, potassium ferricyanide, was used as the redox probe of the imprinted film modified electrodes in solutions containing analyte. The imprinted cavities were used as access holes for potassium ferricyanide. RAC can occupy the imprinted cavities in the imprinted PoPD film, and block electron transfer of redox probe, resulting in a decrease in current. This decrease in current was then applied to quantify RAC concentrations.
3.2 Formation of the imprinted film
The electropolymerization of imprinted PoPD film was carried out in HAc–NaAC electrolyte solution containing 30 mM oPD, and 10 mM RAC by the scanning potential between −1.0 V and +1.0 V for 10 consecutive cycles at a scan rate of 50 mV s−1. Fig. 1 shows representative cyclic voltammograms for electropolymerization process of PoPD on the AuNPs–IL–GR/GCE surface in the presence (A)/absence (B) of RAC. The polymerization process is an irreversible oxidation process, and a strong oxidation peak at ∼+0.4 V and a small reduction peak at ∼−0.6 V were clearly observed on the first scan. Then, both the oxidative and reduction peak decreased dramatically under continuous cyclic scan. After ten cycles of CV scanning, the peak currents almost approached zero as indicated with the arrows in Fig. 1, indicating that the MIP membranes were polymerized onto the surface of the modified electrode. The decrease of the peak currents seems to be related with the continual formation of the MIP membrane that hinders oPD monomer further access to the surface of the AuNPs–IL–GR/GCE. No significant difference was observed between the cyclic voltammograms obtained in the presence of RAC and in its absence during the polymerization. These results demonstrate that RAC does not have electroactivity in the potential range chosen for the polymerization and its structure was not electrochemically altered during electropolymerization.In addition, similar cyclic voltammograms of the electropolymerization processes of oPD on the AuNPs–IL–GR/GCE surface in the absence of RAC had also been observed. Meanwhile, there was no difference in the cyclic voltammograms obtained in the presence/absence of RAC template, indicating that RAC does not exhibit any electroactivity in the potential range chosen for the polymerization and its structure was not electrochemically altered during electropolymerization.
3.3 Surface morphology of various modified electrode
The TEM and AFM images of graphene are shown in Fig. 2A and B, respectively. It is found that graphene sheets were slightly wrinkled and folded on ultrathin carbon membrane, a result of strong surface tension between individual graphene sheet. The well-defined diffraction spots of selected area electron diffraction (SAED, inset of Fig. 2A) pattern confirmed the crystalline structure of the graphene. In terms of atomic force microscopy (AFM) (Fig. 2B), the apparent height of graphene was found to be ∼0.8 nm. Based on TEM and AFM results, it can be concluded that the graphene are well-dispersed in solvent, and crystallined.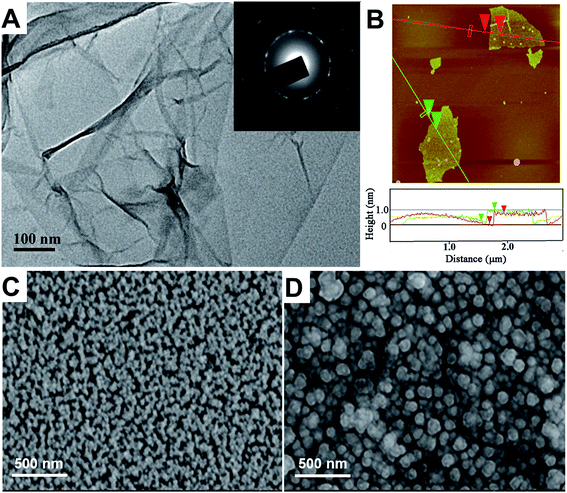 | ||
| Fig. 2 TEM (A) and AFM (B) images of graphene and the typical SEM images of the AuNPs–IL–GR/GCE (C) and the imprinted PoPD/AuNPs–IL–GR/GCE (D). | ||
In order to confirm whether imprinted PoPD had been deposited onto the AuNPs–IL–GR/GCE surface, the morphologies of the AuNPs–IL–GR/GCE before and after the electropolymerization of oPD were observed under scanning electron microscope (SEM). Closely packed AuNPs were observed on the electrode surface in Fig. 2C, and the surface became rough and uneven after the formation of MIPs in Fig. 2D. These results indicate that MIP was deposited on the AuNPs–IL–GR, and its rough surface provided a large surface area on the modified electrode for recognition of target molecules.
3.4 Electrochemical characterization of modified electrode
Cyclic voltammetric (CV) experiments of modified electrodes were performed in 5.0 mmol L−1 K3[Fe(CN)6] and 0.1 M KCl solution from −1.0 V to +1.0 V (vs. SCE) at a scan rate of 50 mV s−1 as shown in Fig. 3A. It can be found that that the current of IL–GR modified electrode (curve b) was higher than that of a bare GCE (curve a), which was attributed to the prominent electron transfer ability of IL–GR. When AuNPs was loaded on the IL–GR surface, the peak current was further increased (curve c), exhibiting the excellent conductivity of AuNPs. In contrast, electropolymerization of oPD on the surface of AuNPs–IL–GR/GCE, led to an obvious decrease of the peak current (curve d) owing to the formation of an electron blocking layer. After the elution of template, the peak current (curve e) was recovered but still less than that of AuNPs–IL–GR/GCE.EIS was further used to determine the electron transfer abilities of the modified electrodes and the corresponding typical Nyquist plots are exhibited in Fig. 3B. The semicircle diameter at higher frequencies corresponds to the electron-transfer resistance (Ret), and the linear part at lower frequencies corresponds to the diffusion process. It was observed that IL–GR (curve b) and AuNPs–IL–GR (curve c) displayed two almost straight lines in the Nyquist plot and the slope of lines increased dramatically, indicating that there was improved diffusion of ferricyanide toward the electrode surface. The Ret of bare electrode (curve a) increased indicated that the GR and AuNPs–IL–GR composites were excellent electric conducting materials, which can form high electron conduction pathways between the electrode and electrolyte to accelerate the electron transfer. After coating with the MIP/AuNPs–IL–GR, the Ret increased (curve d), indicating that the MIPs was successfully composited on the surface. The MIPs formed an additional barrier on the surface of electrode to block the electron exchange between the solution and the electrode. After removing the template, the Ret decreased (curve e), indicating that the template was successfully removed and some cavities formed, which facilitated the electron exchange between the redox.
DPV current response of bare GCE, AuNPs–IL–GR/GCE, MIP/AuNPs–IL–GR/GCE before removal of RAC, and MIP/AuNPs–IL–GR/GCE after removal of RAC are shown in Fig. 3C. After the electropolymerization of oPD in the presence of RAC on the AuNPs–IL–GR/GCE surface, a dramatical decrease in peak current (from curve c to curve d) was observed because the modified PoPD film has partly blocked reactant access to the electrode surface. An apparent increase in peak current was then observed after the template removal step (curve e), indicating the successful generation of imprinted cavities.
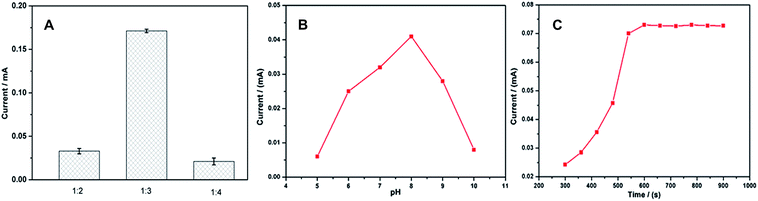
3.5 Optimization of the experimental parameters of the MIP-sensor
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. While the template–monomer ratio is 1
3. While the template–monomer ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the current decreases, probably because the amount of monomer is too much to remove the template molecules, leading to fewer number of recognition sites in the MIP film. Thus, the ratio of 1
4, the current decreases, probably because the amount of monomer is too much to remove the template molecules, leading to fewer number of recognition sites in the MIP film. Thus, the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was used for the electropolymerization.
3 was used for the electropolymerization.
3.6 Performance of the MIP-sensor
Under the optimal experimental conditions described above, the analytical performance of the electrochemical sensor to RAC was examined. It showed that the reductive peak current at 0.15 V was linearly proportional to the RAC concentration in the range of 10 to 5000 μg L−1 in Fig. 5. The linear regression equation was y = −0.0303x + 0.2265, with a correlation coefficient of 0.9732. The detection limit was estimated to be 0.46 μg L−1 (defined as S/N = 3). The response current of the NIP-sensor did not change significantly with the changes in RAC concentration, which indicates that the proposed sensor has excellent specificity. | ||
| Fig. 5 DPV of the sensor incubated with different concentrations of RAC (a–g: 0, 10, 50, 100, 500, 1000, 5000 μg L−1). The inset shows the linear relationship between the peak currents and RAC. | ||
The RAC detection performance with graphene oxide modified GCE, RAC-imprinted film modified gold electrode, multi-wall carbon nanotubes and molecularly imprinted membranes modified screen-printed electrode are summarized in Table 1. The present sensor provided a broader linear range and a lower detection limit for RAC analysis than other methods. Hence, MIP/AuNPs–IL–GR composite modified electrode was confirmed to be efficient electrochemical sensors for rapid determination of RAC.
| Electrochemical sensors | Detection limit (μg L−1) | Linear range (μg L−1) | References |
|---|---|---|---|
| a Graphene oxide modified glassy carbon electrode.b RAC-imprinted film modified gold electrode.c Multi-wall carbon nanotubes and molecularly imprinted membranes modified screen-printed electrode. | |||
| GO-GCEa | 17.00 | 25.00–1000.00 | 12 |
| MIP/gold electrodeb | 8.04 | 67.57–472.96 | 27 |
| MWCNT/MIM/SPEc | 2.03 | 6.76–67.57 | 7 |
| MIP/AuNPs–GR/GCE | 0.46 | 10.00–5000.00 | This work |
3.7 Selectivity of the electrochemical MIP-sensor
In order to verify the selectivity of the electrochemical sensor to RAC, current responses were tested in the presence of some analogs such as CL and SAL. The experimental results showed that very slight changes in peak current response of the MIP-sensor were observed for these interfering substances at the same concentrations of CL and SAL as shown in Fig. 6, but this gentle change did not appreciably detract from the excellent selectivity of the sensor. However, the current response of the non-imprinted sensor changed very slightly at the same concentration of all the compounds mentioned. It can be concluded that the imprinted sensor is highly selective for RAC. These observed results can be explained by the presence of suitable molecular complementary cavities (Scheme 1) and unique binding, which resulted from hydrogen bonds as well as weak interactions between the imprinted sites and RAC molecules. Furthermore, the specific adsorption of RAC molecules into the MIP film and the selective binding within the film attributed to the porosity of imprinted sensor.44 Thus, the selectivity of the electrochemical MIP-sensor and recognition of the specific target molecules were found to be satisfactory. | ||
| Fig. 6 Selective recognition of the imprinted and non-imprinted sensors for detecting RAC and its analogues at the optimal conditions. The concentration of RAC, SAL, and CL was 1000 μg L−1. | ||
3.8 Reproducibility and stability of the electrochemical sensor
The reproducibility of the electrochemical sensor was investigated by testing the detection of 1000 μg L−1 RAC at optimal conditions on five separate occasions using different electrodes. Results showed that the relative SD was 2.17%, indicating good reproducibility. Additionally, when the sensor was used to detect 1000 μg L−1 of RAC on ten occasions, the relative SD was 1.10%.The electrochemical sensor was stored in PBS at <4 °C, and the current responses of RAC (1000 μg L−1) were measured every week. The response decreased by 5.64% and 5.70% of the initial response after being stored for one and two weeks, respectively. About 85% of the original responses were retained after one month, indicating that the sensor had good long-term stability. Moreover, the sensors could be used for 16 subsequent RAC detection cycles after being regenerated.
3.9 Preliminary analysis of real samples
The MIP-sensor was applied to determine RAC in swine urine samples which were collected from a local livestock farm for practical application. To prepare these samples, specific concentrations of RAC were added to the swine urine, and 2 mL was extracted and added to an equal volume of ethyl acetate. The mixture was gently agitated and left to stand until stratification. The supernatant was then collected and dried with nitrogen. Finally, 2 mL of PBS (pH 8.0) was added to dissolve the residue. All samples were stored at 4 °C prior to analysis. MIP-sensor was used for determining the recoveries of three different spiked concentrations of RAC in blank swine urine samples. As shown in Table 2, recoveries obtained using this method were 83–112.2%, and the relative standard deviation (RSD) was varied from 2.7% to 4.1%, which indicated the sensor could detect RAC in real urine samples without complicated treatment.| Samples | Added (μg L−1) | Found (μg L−1) | Recovery (%, n = 3) | RSD (%) |
|---|---|---|---|---|
| 1 | 50 | 41.5 | 83.0 | 2.7 |
| 2 | 100 | 112.2 | 112.2 | 4.1 |
| 3 | 1000 | 967.0 | 96.7 | 3.6 |
4. Conclusion
In this study, a sensitive electrochemical MIP-sensor for the determination of RAC was successfully constructed by electrodepositing MIP on a nanocomposite modified electrode. The resultant sensor demonstrated that integration of AuNPs–IL–GR into the molecular imprinting electropolymerization process can improve the performance of imprinted electrodeposited membrane, while the MIP improve the selectivity. Under optimized conditions, good linearity was achieved in the range of 10 to 5000 μg L−1, the limits detection of RAC was 0.46 μg L−1. This work provides a promising substitute of antibodies, and demonstrate a high performance electrochemical MIP-sensor for the determination of RAC.Acknowledgements
This work was supported by National Natural Science Foundation of China (Contact No. 31471654), and the National Key Technology R&D Program for the 12th five-year plan (2014BAD13B05-05).References
- C. Crescenzi, S. Bayoudh, P. Cormack, T. Klein and K. Ensing, Anal. Chem., 2001, 73, 2171–2177 CrossRef CAS PubMed.
- Y. Xiong, M. Gower, C. Li, C. Elmore, G. Cromwell and M. Lindemann, Meat Sci., 2006, 73, 600–604 CrossRef CAS PubMed.
- E. I. Shishani, S. C. Chai, S. Jamokha, G. Aznar and M. K. Hoffman, Anal. Chim. Acta, 2003, 483, 137–145 CrossRef CAS.
- L. Watkins, D. Jones, D. Mowrey, D. Anderson and E. Veenhuizen, J. Anim. Sci., 1990, 68, 3588–3595 CAS.
- G. Brambilla, T. Cenci, F. Franconi, R. Galarini, A. Macrı, F. Rondoni, M. Strozzi and A. Loizzo, Toxicol. Lett., 2000, 114, 47–53 CrossRef CAS PubMed.
- P. Zuo, Y. Zhang, J. Liu and B.-C. Ye, Talanta, 2010, 82, 61–66 CrossRef CAS PubMed.
- H. Zhang, G. Liu and C. Chai, Sens. Actuators, B, 2012, 168, 103–110 CrossRef CAS.
- A. Blomgren, C. Berggren, A. Holmberg, F. Larsson, B. Sellergren and K. Ensing, J. Chromatogr. A, 2002, 975, 157–164 CrossRef CAS PubMed.
- M. Hernandez-Carrasquilla, Anal. Chim. Acta, 2000, 408, 285–290 CrossRef CAS.
- M. Nielen, J. Lasaroms, M. Essers, J. Oosterink, T. Meijer, M. Sanders, T. Zuidema and A. Stolker, Anal. Bioanal. Chem., 2008, 391, 199–210 CrossRef CAS PubMed.
- W. Wang, Y. Zhang, J. Wang, X. Shi and J. Ye, Meat Sci., 2010, 85, 302–305 CrossRef CAS PubMed.
- C. Wu, D. Sun, Q. Li and K. Wu, Sens. Actuators, B, 2012, 168, 178–184 CrossRef CAS.
- M. Wang, M. Kang, C. Guo, S. Fang, L. He, C. Jia, G. Zhang, B. Bai, W. Zong and Z. Zhang, Electrochim. Acta, 2015, 182, 668–675 CrossRef CAS.
- F. Yang, P. Wang, R. Wang, Y. Zhou, X. Su, Y. He, L. Shi and D. Yao, Biosens. Bioelectron., 2016, 77, 347–352 CrossRef CAS PubMed.
- F. L. Dickert, P. Lieberzeit, S. G. Miarecka, K. J. Mann, O. Hayden and C. Palfinger, Biosens. Bioelectron., 2004, 20, 1040–1044 CrossRef CAS PubMed.
- S. A. Piletsky and A. P. Turner, Electroanalysis, 2002, 14, 317–323 CrossRef CAS.
- B.-Y. Huang, Y.-C. Chen, G.-R. Wang and C.-Y. Liu, J. Chromatogr. A, 2011, 1218, 849–855 CrossRef CAS PubMed.
- Y. Sueyoshi, C. Fukushima and M. Yoshikawa, J. Membr. Sci., 2010, 357, 90–97 CrossRef CAS.
- P.-Y. Chen, R. Vittal, P.-C. Nien, G.-S. Liou and K.-C. Ho, Talanta, 2010, 80, 1145–1151 CrossRef CAS PubMed.
- L. Meng, X. Qiao, J. Song, Z. Xu, J. Xin and Y. Zhang, J. Agric. Food Chem., 2011, 59, 12745–12751 CrossRef CAS PubMed.
- S. Mei, D. Wu, M. Jiang, B. Lu, J.-M. Lim, Y.-K. Zhou and Y.-I. Lee, Microchem. J., 2011, 98, 150–155 CrossRef CAS.
- G. Cirillo, M. Curcio, O. I. Parisi, F. Puoci, F. Iemma, U. G. Spizzirri, D. Restuccia and N. Picci, Food Chem., 2011, 125, 1058–1063 CrossRef CAS.
- L.-J. Kong, M.-F. Pan, G.-Z. Fang, X.-l. He, Y.-k. Yang, J. Dai and S. Wang, Biosens. Bioelectron., 2014, 51, 286–292 CrossRef CAS PubMed.
- H. Liu, G. Fang and S. Wang, Biosens. Bioelectron., 2014, 55, 127–132 CrossRef CAS PubMed.
- H. Liu, D. Liu, G. Fang, F. Liu, C. Liu, Y. Yang and S. Wang, Anal. Chim. Acta, 2013, 762, 76–82 CrossRef CAS PubMed.
- R. Lei, C. Guo, H. Xiong, C. Dong, X. Zhang and S. Wang, Electroanalysis, 2014, 26, 1004–1012 CrossRef CAS.
- L.-J. Kong, M.-F. Pan, G.-Z. Fang, K. Qian and S. Wang, Anal. Bioanal. Chem., 2012, 404, 1653–1660 CrossRef CAS PubMed.
- A. R. bin Mohd Yusoff and S. A. Shuib, Electrochim. Acta, 2011, 58, 417–421 CrossRef.
- H. Peng, C. Liang, A. Zhou, Y. Zhang, Q. Xie and S. Yao, Anal. Chim. Acta, 2000, 423, 221–228 CrossRef CAS.
- M. Mazloum-Ardakani, M. Sheikh-Mohseni and A. Benvidi, Electroanalysis, 2011, 23, 2822–2831 CrossRef CAS.
- C. Malitesta, I. Losito and P. G. Zambonin, Anal. Chem., 1999, 71, 1366–1370 CrossRef CAS PubMed.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2009, 110, 132–145 CrossRef PubMed.
- Y. Shao, J. Wang, H. Wu, J. Liu, I. A. Aksay and Y. Lin, Electroanalysis, 2010, 22, 1027–1036 CrossRef CAS.
- J. R. Taylor, M. M. Fang and S. Nie, Anal. Chem., 2000, 72, 1979–1986 CrossRef CAS PubMed.
- W. Bai, H. Huang, Y. Li, H. Zhang, B. Liang, R. Guo, L. Du and Z. Zhang, Electrochim. Acta, 2014, 117, 322–328 CrossRef CAS.
- J. Li, Y. Shen, Y. Zhang and Y. Liu, Chem. Commun., 2005, 360–362 RSC.
- T. D. Ho, C. Zhang, L. W. Hantao and J. L. Anderson, Anal. Chem., 2013, 86, 262–285 CrossRef PubMed.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- A. P. Abbott, P. M. Cullis, M. J. Gibson, R. C. Harris and E. Raven, Green Chem., 2007, 9, 868–872 RSC.
- P. Hapiot and C. Lagrost, Chem. Rev., 2008, 108, 2238–2264 CrossRef CAS PubMed.
- Y. Zhang, T.-F. Kang, Y.-W. Wan and S.-Y. Chen, Microchim. Acta, 2009, 165, 307–311 CrossRef CAS.
- M. Riskin, R. Tel-Vered, T. Bourenko, E. Granot and I. Willner, J. Am. Chem. Soc., 2008, 130, 9726–9733 CrossRef CAS PubMed.
- I. Rubinstein, J. Rishpon, E. Sabatani, A. Redondo and S. Gottesfeld, J. Am. Chem. Soc., 1990, 112, 6135–6136 CrossRef CAS.
- H. Chasta and R. N. Goyal, Talanta, 2014, 125, 167–173 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11999a |
| This journal is © The Royal Society of Chemistry 2016 |