Significantly enhanced dewatering performance of drinking water sludge from a coagulation process using a novel chitosan–aluminum chloride composite coagulant in the treatment of cyanobacteria-laden source water†
Chunxia Maa,
Haiyan Pei*ab,
Wenrong Huab,
Juan Chenga,
Hangzhou Xua and
Yan Jina
aSchool of Environmental Science and Engineering, Shandong University, Jinan 250100, China. E-mail: haiyanhup@126.com; Fax: +86-531-88392983; Tel: +86-531-88392983
bShandong Provincial Engineering Center on Environmental Science and Technology, Jinan, China
First published on 20th June 2016
Abstract
The enhanced dewatering performance and the fate of cyanobacterial cells in the filtration of cyanobacteria-laden sludge, generated by a coagulation process using a novel composite chitosan–aluminum chloride (CTSAC) coagulant, were systemically studied. Two other cyanobacteria-laden sludge, aluminum chloride (AC) sludge and chitosan (CTS) sludge, were also studied to compare dewater performance with CTSAC sludge. Results showed that the dewatering process did not cause cell lysis and microcystins (MCs) release. The level of MCs and extracellular organic matter (EOM) in the filtrate were decreased by adsorption and sieving onto the cake layer formed on the membrane, but dewatering at high vacuum pressure reduced the rejection efficiency. The sludge from the coagulation process using the CTSAC composite displayed better sludge dewaterability and obtained a better quality of filtrate (fewer MCs and EOM) than those from AC and CTS coagulation processes independently. A three-dimensional excitation–emission matrix (EEM) fluorescence measurement indicated that protein-like substances in soluble extracellular polymeric substances (EPS) played a negative role on cyanobacteria-laden sludge dewatering. In addition, CTSAC sludge showed a more compact structure and larger floc sizes than AC sludge and CTS sludge for a strong improvement in the charge neutralization and bridge ability of AC by combining CTS in the composite coagulant. It was further observed that floc size played a more significant role on sludge dewaterability than the degree of compactness. Overall, the preferable dewater performance of CTSAC sludge demonstrated the CTSAC composite coagulant has great potential for the treatment of cyanobacteria-laden source water.
1. Introduction
The frequent episodes of cyanobacterial blooms have become a worldwide problem for drinking water treatment. M. aeruginosa, one of the typical cyanobacteria found in fresh water, releases the most prevalent toxins called microcystins (MCs) that can cause illness or harm human health.1,2 Furthermore, the algal organic matter (AOM) secreted by M. aeruginosa serves as a precursor to form disinfection by-products (DBPs) during chlorination that also carry a health risk.1 The majority of AOM including MCs contained within M. aeruginosa cells are intracellular organic matter (IOM), and the metabolites secreted into the environment are extracellular organic matter (EOM).1 The IOM can be released into the water due to cell ageing and/or induced cell membrane damage.2 Unlike IOM, dissolved EOM are ineffectively removed by conventional clarification methods.2 Currently, most researches have focused on effective removal of cyanobacteria from the water phase during drinking water treatments, such as coagulation, flocculation and sedimentation. However, the potential danger of cyanobacterial cells transferred into the solid phase, especially the drinking water sludge has been neglected.There are large amounts of drinking water sludge produced from conventional coagulation processes: up to 7% of the total net volume of produced water.3 These characteristics of drinking water sludge will bring about serious pollution to resource waste if the sludge is discharged without disposal.3 Thus the treatment of drinking water sludge is attracting more and more attention.3–5 Sludge dewatering is the key process in the treatment of the sludge as it reduces the quantity of the final waste product and, thus, the cost of transporting sludge to the final disposal site.4,6 The most common way for sludge dewatering is mechanical dewatering, while filtration has become an attractive technology to reach desired removal efficiency.4 Previous literature focusing on sludge treatment suggested that the dewatering and filtration process are closely associated with the sludge properties, which are also associated with the quality of raw water and the nature of the coagulant used in the process.
The composite coagulant CTSAC is an organic/inorganic polymer composite coagulant with high efficiency in cyanobacteria removal. Our previous research found that at low dosage, CTSAC coagulant could not only remove M. aeruginosa without cell lysis, but also adsorb a significant amount of EOM, especially extracellular MCs, while the individual CTS and AC coagulants were not as effective as the combined one.2,7,8 However, until now, there is still a lack of information about the properties of cyanobacteria-laden sludge from the enhanced coagulation process using composite coagulant, such as zeta potential of flocs and dewatering ability of sludge. Furthermore, although the M. aeruginosa cells can be removed without causing cell lysis from composite coagulation process, the existed coagulants and various mechanical actions of dewatering process both could cause external stress on cyanobacterial cells, thus cell lysis can still occur in the sludge dewatering process, and release a large amount of EOM especially MCs metabolites into the sludge supernatant.8 Therefore, it is necessary to elucidate the effects of the dewatering process on cyanobacterial cells and EOM in cyanobacteria-laden sludge and try to reduce the MCs and EOM release.
In this study, we provide a comprehensive insight into the dewaterability and the fate of cyanobacteria cells of cyanobacteria-laden CTSAC sludge during the dewatering process. AC and CTS coagulation generated cyanobacteria-laden sludge acted as control groups. Different vacuum pressures were introduced to investigate the mechanical effect on cyanobacteria-laden sludge dewatering. In addition, sludge size and compaction level, and three-dimensional excitation–emission matrix (EEM) fluorescence spectra of extracellular polymeric substances (EPS) were analyzed to get mechanism understanding of the sludge dewatering.
2. Experimental procedures
2.1. Materials
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, D.D = 95%) in 100 mL of 1.0% acetic acid solution and stirred overnight. The CTSAC composite coagulant was prepared by adding an amount of AC into CTS stock solution with continuous stirring for 24 h to obtain a CTSAC mixed solution with composite concentration of 1.3 mg mL−1 CTS plus 3.75 mg mL−1 AC.
000, D.D = 95%) in 100 mL of 1.0% acetic acid solution and stirred overnight. The CTSAC composite coagulant was prepared by adding an amount of AC into CTS stock solution with continuous stirring for 24 h to obtain a CTSAC mixed solution with composite concentration of 1.3 mg mL−1 CTS plus 3.75 mg mL−1 AC.2.2. Coagulation experiment
Coagulation experiments were performed in a program-controlled jar test apparatus (ZR4-6, Zhongrun Water Industry Technology Development Co. Ltd., China) at 25 ± 2 °C. A resuspended M. aeruginosa water sample of 1000 mL was used for each coagulation experiment. The pH of the samples was adjusted to about 8.4 by adding 0.1 M NaOH or HCl to keep consistent with our previous study.8 For the AC coagulation experiment, 15 mg L−1 AC was added when the rapid mixing (250 rpm) started. After 1 min rapid mixing, the stirring was slowed to 20 rpm for 20 min.2 As for the CTS coagulation experiment, the coagulation process was simulated firstly by rapid mixing at 215 rpm for 1 min after addition of 7.5 mg L−1 CTS, followed by slow stirring at 16 rpm for 9 min.7 The CTSAC composite coagulation was started by addition of CTSAC (2.6 mg L−1 CTS plus 7.5 mg L−1 AC) into the water sample; then coagulation was conducted by rapid mixing at 250 rpm for 2 min followed by 20 rpm for 20 min.8 The coagulation dosages and mechanical actions in AC, CTS and CTSAC coagulation processes were optimal respectively, which were confirmed by previous studies.2,7,8 After coagulation, all water samples were left to stand for 30 min to separate the supernatant and sludge. The unfiltered supernatant was used for zeta potential and chlorophyll a auto-fluorescence measurement, and the samples were filtered through glass fiber membranes (0.45 μm) for analysis of extracellular MCs, K+ release, UV254, protein, and polysaccharide.2.3. Vacuum filtration experiment on the cyanobacteria-laden sludge
The dewatering process of cyanobacteria-laden drinking water sludge is using a vacuum gauge. Cyanobacteria-laden sludge (50 mL) produced by the different coagulations remained after settling and removing the supernatant. The sludge was mixed briefly and 2 mL was abstracted into a syringe. In the vacuum filtration process, a solid phase extraction device with a vacuum gauge (Tianjin Automatic Science Instrument Co., Ltd., China) was fitted with a filter. A 0.45 μm aqueous cellulose acetate (CA) membrane (Membrane Solutions, USA) with a surface area of 13 mm was employed in the experiments. The filtrate was removed from the bottom of the collection tube for MCs and EOM analysis of samples for each of the different vacuum pressures.2.4. EPS extraction
Centrifugation procedures for EPS fractionation of sludge samples and algae solution are detailed elsewhere.9,10 Briefly, the sludge samples were firstly centrifuged at 4000g for 15 min at 4 °C. Then, the supernatant was filtered through a 0.45 μm glass fiber membrane to obtain the soluble-EPS solution (Taoyuan, China). The sludge pellet from the centrifuge tube and sludge collected on the surface of the 0.45 μm glass fiber membrane were collected and re-suspended with 0.6% NaCl solution to prevent cell damage.1 The re-suspended solution was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min at 4 °C, and subsequently filtered through a 0.45 μm glass fiber membrane to obtain the bound-EPS solution. A high-speed refrigerated centrifuge was utilized to centrifuge the sludge (GL-21B, Anting, China).
000g for 15 min at 4 °C, and subsequently filtered through a 0.45 μm glass fiber membrane to obtain the bound-EPS solution. A high-speed refrigerated centrifuge was utilized to centrifuge the sludge (GL-21B, Anting, China).
2.5. Analytical methods
A Malvern Mastersizer 2000 laser diffraction instrument (Malvern, UK) was used to measure the particle size distribution before and after coagulation. UV254 absorbance was measured using a UV spectrophotometer (U-3010, Hitachi Co., Japan) according to standard methods of analysis.11 The bicinchoninic acid (BCA) reagent (Shanghai Sangon Biological Engineering Technology & Services Co., Ltd) was used to measure the protein content following a modified Lowry method.12 The polysaccharide concentration was determined by the phenol-sulphuric acid method.13 All analyses were conducted in triplicate with standard errors less than 5%.
3. Results and discussion
3.1. The comparison of sludge dewaterability
The filtration rates of cyanobacteria-laden sludge treated with different coagulants are shown in Fig. 1. Compared with raw M. aeruginosa culture, AC sludge, and CTS sludge, the composite coagulant sludge gave a markedly faster filtration rate at the same vacuum pressure. It could be found the filtration rate of each sample increased as the increase of vacuum pressure, and the filtration rate at −0.9 bar was larger than those at −0.5 bar, −0.6 bar, −0.7 bar and −0.8 bar for each of the sludge samples. The increased filtration rate of composite coagulant sludge from −0.5 bar to −0.9 bar was higher than other samples.With increasing vacuum, a higher filtration rate is expected due to the pressure difference between the two sides of the membrane. For the raw M. aeruginosa suspension without pretreatment, the lower filtration rate was predominantly due to pore blocking caused by relatively small particles in the water.14 It has been widely reported the fine colloids and EOM adsorbed and plugged into the cake layer pore structures to form cake layer on the membrane surface would determine the membrane resistance and flux decrease.14,15 In this case, the increased vacuum led to more increase of filtration rate in filtration of CTSAC composite coagulation, in comparison with CTS and AC. It indicates that the CTSAC sludge had less resistance during the dewatering process.
The CST of raw M. aeruginosa suspension and different coagulation-generated sludge are listed in Table 1. The CST of raw M. aeruginosa was similar to that of AC sludge, but longer than that of CTS coagulation sludge. And the CST of the composite coagulation sample was the lowest followed by AC and CTS coagulation sludge. This finding suggests that the dewaterability of composite sludge was much better than the other samples.
| Sludge sources | Characteristics of the sludge samples | ||
|---|---|---|---|
| pH | Zeta potential (mV) | CST (s) | |
| a M. a: Microcystis aeruginosa suspension, AC: aluminum chloride, CTS: chitosan. | |||
| M. a | 8.42 | −33.5 ± 0.7 | 7.23 |
| AC sludge | 8.01 | −17.9 ± 0.4 | 5.83 |
| CTS sludge | 6.79 | 20.2 ± 1.0 | 2.33 |
| CTSAC sludge | 7.13 | 1.25 ± 0.1 | 0.70 |
The zeta potential is an important parameter in influencing sludge dewaterability.9 With decreasing surface charge associated with elevating zeta potential close to zero, the sludge can aggregate and settle quickly, so that it is dewatered more easily. This theory is well supported by our findings, in which the composite coagulation sludge showed the best dewaterability with a zeta potential of 1.25 ± 0.1 mV, which is closer to zero than the other samples (Table 1). Zhen et al. also confirmed that the dewaterability of waste activated sludge was greatly enhanced when the zeta potential increased from −18 mV at the initial stage to close to −0.4 mV after Fe(II)–activated persulfate oxidation.9
3.2. Effect of the dewatering process on cell integrity
Cyanobacterial cell integrity during sludge filtration is crucially important because the shear stresses developed at the membrane surface or from vacuum pumping may cause cell damage, with subsequent release of intracellular MCs and IOM into the permeate. It has been shown that the release of K+ can indicate the damage of the M. aeruginosa cell membrane because K+ is absorbed into the vacuole of M. aeruginosa cell and stored as enzyme activator.16 Furthermore, the CA membrane used in this study cannot retain the dissolved K+ ions (data not shown). It can be observed in Table 2 that the concentration of K+ in the coagulated solution before and after filtration both at −0.5 bar and at −0.9 bar vacuum was similar, and that no apparent release of K+ was observed. Chlorophyll a, as a single form of intracellular chlorophyll in M. aeruginosa cells, showed red fluorescence which indicated that the cells were in normal cell viability as shown in Fig. S1.† The variation of red fluorescence is associated with the presence of chlorophyll a in M. aeruginosa cells, and the decrease in chlorophyll a would lead to the decline of the red fluorescence of cells.17 As shown in Fig. S1,† the red fluorescence of M. aeruginosa cells was strong and uniform for all the coagulated samples before and after filtration both at −0.5 bar and at −0.9 bar vacuum. These indicated that M. aeruginosa cells were alive and no obvious cell damage occurred during cyanobacteria-laden AC sludge, CTS sludge and CTSAC composite sludge dewatering.| Sample | K+ (mg L−1) | ||
|---|---|---|---|
| Before filtration | Filtration at −0.5 bar | Filtration at −0.9 bar | |
| a M. a: Microcystis aeruginosa suspension, AC: aluminum chloride, CTS: chitosan. | |||
| M. a | 4.52 ± 0.43 | 4.61 ± 0.25 | 4.48 ± 0.25 |
| AC sludge | 4.49 ± 0.71 | 4.55 ± 0.31 | 4.64 ± 0.29 |
| CTS sludge | 4.58 ± 0.42 | 4.65 ± 0.45 | 4.51 ± 0.59 |
| CTSAC sludge | 4.6 ± 0.25 | 4.35 ± 0.25 | 4.29 ± 0.25 |
This is related to the fact that the critical pressure of cyanobacteria is up to 6 bar (ref. 18) and the pressure of vacuum filtration (<−0.9 bar) is lower than this critical value and therefore has no damaging effects on the cyanobacterial cells.
3.3. Impact of the dewatering process on extracellular MCs
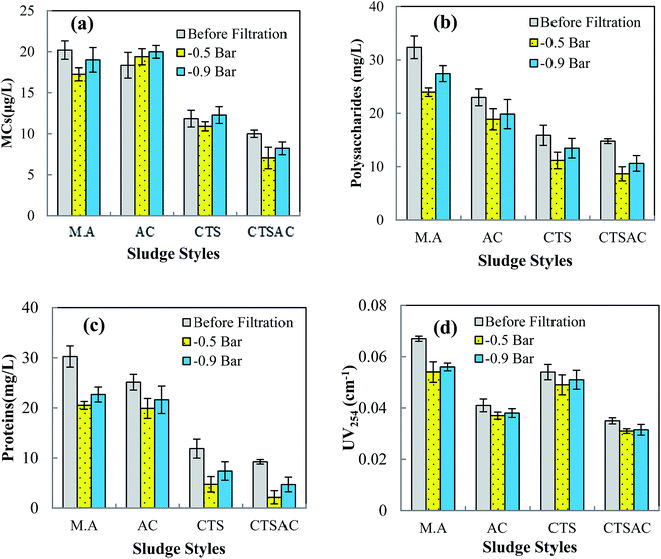
After vacuum filtration, the concentration of raw MCs decreased from 20.20 μg L−1 to 17.25 μg L−1 at −0.5 bar and 19.01 μg L−1 at −0.9 bar (Fig. 2(a)). The MCs adsorption capacity of AC was quite small, while the CTS and CTSAC composite had effective MCs adsorption ability, which is consistent with our previous studies.2,7,19 Result showed that for AC sludge, the MCs level of the filtrate was higher than that in treated water (coagulation supernatant). For CTS sludge, the MCs concentration slightly declined in permeate. And for the CTSAC composite sludge, the MCs concentration of the filtrate was reduced to 7.05 μg L−1 at −0.5 bar and 8.23 μg L−1 at −0.9 bar, respectively. These results indicated the filtration dewatering of CTSAC composite sludge could result in effective MCs removal.The solutes rejection mechanisms during membrane filtration of sludge are widely recognized.20 Firstly, the solutes could be efficiently rejected by membrane when the solutes are larger than the membrane pores size, i.e. a sieving mechanism. Adsorption of solutes into the membrane pores and surfaces is considered as the second mechanism for solute rejection. Thirdly, after the sludge flocs are collected onto the membrane, the EPS, soluble organics, and colloidal particles are sieved or adsorbed onto the cake layer formed over the membrane surface. Considering that the MCs are relatively hydrophobic compounds with a molecular weight of about 985–1024 Da, which is much below the cut-off of the CA hydrophilic membrane,21 the contribution of the membrane sieving for the MCs rejection is limited. On the other hand, it has been shown that the hydrophilic CA membrane presented low adsorption ability to MCs.22,23 Consequently, the MCs rejection discrepancy of different sludge mainly depends on how much the MCs are adsorbed and/or sieved onto the cakes layer formed during the different coagulation sludge filtration processes. According to our previous study, the retained coagulation cyanobacteria-laden sludge in this study was enough to form a stable sludge layer to separate the sludge and filtrate during the sludge dewatering process.20 Marshall et al. noted the cake layer formed on the membrane surface could trap some low MW molecules and improve the removal of organic matters during the filtration process. As no obvious cell damage occurred during the filtration process, the increase of MCs in the filtrate of the AC sludge can be attributed to the density of cyanobacterial cells in sludge being much greater than that in raw water and the MCs rejection effect of cake layer formed in the dewatering process of AC sludge was not sufficient to remove the MCs. It can be inferred that the cake layer formed during the filtration of CTSAC composite sludge was more suitable for MCs rejection, thus resulting in the higher reduction of MCs.
3.4. Influence of dewatering process on EOM level
EOM content in the feed and permeate of vacuum filtration obtained at −0.5 bar and −0.9 bar are shown in Fig. 2(b). If raw water was filtered, the concentration of polysaccharide in feed water of 32.35 mg L−1 dropped to 23.95 mg L−1 and 27.93 mg L−1 at −0.5 bar and −0.9 bar vacuum filtration, respectively. The polysaccharide concentration in supernatant water after composite coagulation decreased markedly to 16.78 mg L−1, less than that of using AC coagulation (23.98 mg L−1) and CTS coagulation (17.87 mg L−1). The polysaccharide concentrations in permeate of composite sludge were lowered to 8.91 mg L−1 and 10.9 mg L−1 at −0.5 bar and −0.9 bar vacuum, respectively. At same vacuum pressure, the dewatering of composite sludge removed more polysaccharide than that of AC sludge and CTS sludge. For example at −0.5 bar vacuum, the filtration of composite sludge removed 7.87 mg L−1 polysaccharide while filtration of AC sludge and CTS sludge removed 4.92 mg L−1 and 5.90 mg L−1 polysaccharide, respectively. As shown in Fig. 2(c), the trend for protein was consistent with the data for polysaccharide, with enhanced removal of protein in the CTS and composite coagulation process. Furthermore, the rejection effect of vacuum filtration on protein was enhanced compared to that of polysaccharide. As shown, the filtration of composite sludge removed 8.47 mg L−1 protein at −0.5 bar vacuum while filtration of AC sludge and CTS sludge at −0.5 bar vacuum removed 6.18 mg L−1 and 8.05 mg L−1 protein, respectively.It is known that the EOM of M. aeruginosa with size bigger than 0.45 μm might be low,24 thus the rejection of polysaccharide and protein by membrane sieving played a negligible part in sludge filtration process. According to the studies of Qu et al.14 and Henderson et al.,25 most of the polysaccharides in EOM are located in the hydrophilic fraction. Conversely, proteins are characterized by their hydrophobicity with a hydrophobic fraction more than 60%.14 For CA membrane that is more hydrophilic, the adsorption coefficient of CA membrane to hydrophilic substances was superior compared with hydrophobic substances and the organic matter adsorption ability of CA membrane was relatively small.26 Consequently, the difference in polysaccharides and protein rejection efficiency was mainly due to the degree of adsorption and/or sieving onto the cakes layer deposited on the membrane.
UV254 data reflects organic compounds that have intense absorbance at 254 nm including humic substances and aromatic organic compounds.27 Furthermore, UV254 has been widely applied to indicate the M. aeruginosa produced humic substances and aromatic organic compounds during water treatment processes.27–29 As shown (Fig. 2(d)), CTS coagulation resulted in a lower UV254 content reduction compared to AC coagulation. Composite coagulant was more efficient than CTS in removing UV254 absorbing compounds. For raw UV254, the values in the filtrate were lowered to 0.053 cm−1 and 0.058 cm−1 at −0.5 bar and −0.9 bar, respectively. However, the composite coagulation reduced UV254 values in feed water by 0.035 cm−1, and the contents in the filtrate were lowered to 0.032 cm−1 and 0.034 cm−1 at −0.5 bar and −0.9 bar, respectively. Comparing the fate of the polysaccharide, protein and humic-like substances, more of the polysaccharide and protein substances were removed by the sludge filtration process, whereas the more humic-like substances diffused across the membranes.
This is ascribed to the fact that polysaccharide and protein organic substances in EOM were mainly distributed in the high MW fraction while humic-like substances were distributed in a much lower MW fraction. Thus the reject effect of the cake layer to humic-like substances was lower than for polysaccharide and protein substances. As shown in Fig. 2, higher vacuum (−0.9 bar) could improve the filtration rate, but it also decreased the rejection effect of MCs and EOM. This is due to the high pressure in the filtration procedure that could contribute to either the deflocculation of coagulant absorbed EOM or the exfiltration of intracellular EOM.20
It could be summarized that the application of CTSAC composite coagulant in the coagulation of cyanobacterial-laden water is the optimum choice to improve the removal of secondary pollution in the filtration of cyanobacterial-laden sludge at low vacuum operating pressure.
3.5. EEM fluorescence analysis
Typical EEM fluorescence spectra of soluble and bound extracellular polymeric substances (EPS) and the corresponding fractions extracted from the raw M. aeruginosa suspension and coagulation generated sludge are depicted in Fig. 3 and 4. Four major peaks could be identified from fluorescence spectra of EPS as in other studies.9,14 As illustrated in Fig. 3, the first peak (peak Flu 1) observed at Ex/Em of 270–280/305–310 nm in EEM spectra belonged to protein-like substances.30,31 The second peak located at Ex/Em of 345/435–445 nm (peak Flu 2) and the third peak (Flu 3) found at the Ex/Em of around 275/435–445 nm were ascribed to humic- and fulvic-like substances, respectively (Fig. 3).9,30 The fourth peak (peak Flu 4) occurred at Ex/Em of around 280/350–360 nm, representing dissolved microbial metabolites (Fig. 4).30 The results showed that protein-like substances (represented as peak Flu 1) and humic and fulvic-like substances (represented as peak Flu 2 and Flu 3) were three major substances in the soluble-EPS of M. aeruginosa suspension. For the soluble-EPS of AC sludge, all the three major substances existed but the intensity slightly decreased. For CTS and CTSAC sludge, peak Flu 1 disappeared in the soluble-EPS. And the fluorescence intensity of peak Flu 2 increased in soluble-EPS of CTS sludge compared to raw M. aeruginosa suspension. Compared to soluble-EPS, the locations of peaks were quite consistent in bound-EPS of M. aeruginosa suspension and sludge flocs, apart from a small difference in that peak Flu 2 and peak Flu 3 just appeared in bound-EPS of CTSAC sludge.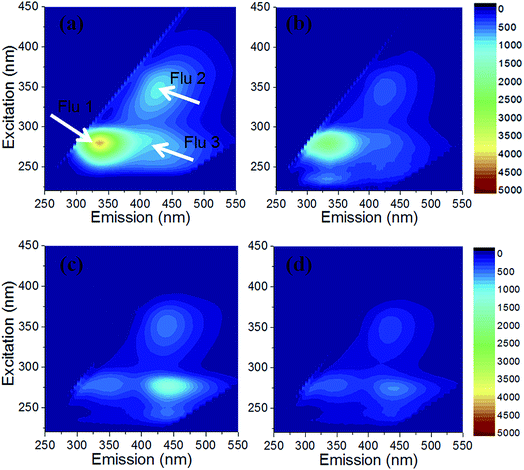 | ||
| Fig. 3 EEM fluorescence spectra of the soluble EPS fractions from the raw Microcystis aeruginosa (M. a) suspension (a), and cyanobacteria-laden AC sludge (b), CTS sludge (c), and CTSAC sludge (d). | ||
 | ||
| Fig. 4 EEM fluorescence spectra of the bound EPS fractions from the raw Microcystis aeruginosa (M. a) suspension (a), and cyanobacteria-laden AC sludge (b), CTS sludge (c), and CTSAC sludge (d). | ||
Decreased CST and increased filtration rate (Table 1) accompanied by reduced fluorescence intensity of protein-like substances in soluble-EPS (Fig. 3) was observed, revealing that the decrease of soluble-EPS favors the enhancement of sludge dewaterability. Liu et al. reported a close relationship between protein-like substances of EPS and sludge compression and dewatering of membrane in MBRs, while Wang et al. also observed that the specific cake resistance increased as protein-like substances rose.32,33 Li et al. noted the existence of soluble-EPS showed a clearly negative influence on dewaterability, while no correlation was found between bound-EPS and dewaterability.34
A large amount of EPS usually contribute to lower sludge dewaterability, and that this may be due to the steric force produced by EPS, which hinders the contact between flocs particles.35 The strong affinity of composite coagulant to the EPS could implement charge neutralization and compress the soluble-EPS, and thus lead to formation of tightly aggregated flocs and improved dewaterability. In addition, sludge dewaterability was largely affected by dissolved macromolecular compounds (proteins and polysaccharides), which can block the filter pores, and increase the resistance in filtration during dewatering of sludge.15,36 The removal of high molecular weight EPS by the composite coagulation process also contributed to the improvement of composite sludge dewaterability.
3.6. Floc size and structure analyses
The M. aeruginosa cells are nearly globose and 1–10 μm in diameter as depicted in Fig. 5. The metabolism of cyanobacterial cells could release some glue that will make the algal cells stick together. Therefore, the cell aggregation between 10 and 300 μm also exists in M. aeruginosa culture. It is notable that the particle size distributions of the sludge were affected remarkably by different types of coagulants. Fig. 5(a) showed the AC sludge had the largest portion of small particles which led to a smallest mean floc size, and the flocs size distribution curve was shifted to the larger size range in CTSAC sludge. According to Fig. 5(b), for AC sludge the medium diameter was only 181.9 μm, which was much smaller than that of CTS sludge (463.0 μm). It can be seen the CTSAC sludge had the largest median floc size which was higher than 549.5 μm. The combined use of AC and CTS can coagulate the majority of the small particles and make the flocs larger. It was observed that the decreased CST (Table 1) correlated well with the increase of sludge size (Fig. 5), which reveals that flocs size is a significant principal factor with regards to sludge dewatering. It also can be noted that the filtration rate of the sludge was closely associated with floc size: the larger the flocs, the higher the filtration rate. The Carman Kozeny equation illustrates that particle size is inversely proportional to specific cake resistance which means that the cake on membrane surface was much more compressible with larger aggregation.37 | ||
| Fig. 5 Flocs size distributions (a) and cumulative volume distributions (b) of raw Microcystis aeruginosa (M. a) suspension and cyanobacteria-laden AC sludge, CTS sludge and CTSAC sludge. | ||
The degree of compactness of the aggregates is also an important parameter affecting the filtration behaviors.15,37 According to Fig. 6, the sludge flocs compact in the following order: CTS sludge < AC sludge < CTSAC sludge. It is noted in the literature that loosely structured flocs generate less resistance for membrane filtration whereas compact flocs result in a cohesively structured cake layer with poor porosity and permeability, and thus have a negative effect on membrane permeability.10 However, it can be inferred from our results that floc size had a more significant effect on sludge dewaterability than their degree of compactness.
 | ||
| Fig. 6 The photomicrographs of raw Microcystis aeruginosa (M. a) suspension (a) and cyanobacteria-laden AC sludge (b), CTS sludge (c) and CTSAC sludge (d) samples. | ||
The mechanism of AC coagulation was a combination of entrapment and charge neutralization, but there is a lack of powerful bonds linking flocs together.38 CTS, which is an economical and nontoxic biomaterial, played a role in charge neutralization and strong adsorption in the formation of flocs.6,7 Furthermore, linking and bridging occurred when the CTS long-chain polymer extended from the formed flocs to attach more colloids.7 Thus larger flocs formed in CTS coagulation than in AC coagulation, which was consistent with Hu's study.38 However, the bridging mechanism also resulted in a relaxed conformation and thus CTS formed looser flocs than AC. With enhanced charge neutralization, polymer bridging and adsorption ability, the composite coagulation removes more EOM during the coagulation process, and leads to more compact and large flocs which are beneficial to sludge filtration and the dewatering process. It has been identified that a high proportion of large particles would lead to shorter sludge layer formation process and the compact flocs deposited over the membrane could form tighter cake layers.20,39 Therefore, the amounts of EOM sieved and/or adsorbed onto the cakes formed during the filtration of CTSAC composite sludge were greater than that of the other sludge.
4. Conclusion
The dewatering performance of cyanobacteria-laden sludge from an enhanced coagulation process using CTSAC composite was systematically studied in this work. The impacts of mechanical actions and chemical effects on the filtration efficiency and filtrate quality during the cyanobacteria-laden sludge volume reduction process were also determined. The following conclusions can be drawn.(1) The vacuum filtration had rejection effects on extracellular MCs and EOM (polysaccharide, protein and humic acid substances) without causing cyanobacteria cell lysis during cyanobacteria-laden sludge dewatering process.
(2) The sludge from the enhanced CTSAC composite coagulation process was of high dewatering ability, sequentially followed by CTS and AC sludge. For CTSAC has good EOM coagulation performance and the cake layer formed by CTSAC sludge was more effective in the EOM rejection, the dewatering of CTSAC cyanobacteria-laden sludge obtained the filtrate with lowest EOM.
(3) High vacuum can improve the filtration rate but also decrease the rejection effect of MCs and EOM because high pressure causes deflocculation of adsorbed MCs and EOM. Overall, for improving filtration efficiency and filtrate quality, and saving energy cost, it is better to choose low vacuum degree pressure in the vacuum filtration process of cyanobacteria-laden sludge.
(4) The floc size played a more significant effect on sludge dewaterability than their degree of compaction degree. The protein-like substances in soluble-EPS was negatively correlated with the dewaterability of cyanobacteria-laden sludge while no clear correlation was observed between bound-EPS and dewaterability.
(5) The reasons for the large particle size, compact structure, low extracellular organic matters and high dewaterability of the sludge from the enhanced CTSAC composite coagulation process were the strong improvement in the charge neutralization and bridge ability of inorganic AC by combining organic CTS coagulant.
Acknowledgements
This work is supported by the Program for New Century Excellent Talents in University of the Ministry of Education of China (Grant No. NCET-12-0341), Natural Science Foundation of China (51478251), the International Cooperation Research of Shandong Province (2011176), Science and Technology Development Project of Shandong Province (2012GHZ30020), the International Science & Technology Cooperation Program of China (2010DFA91150) and National Science Fund for Excellent Young Scholars (51322811). The authors thank Dr James W. Golden of University of California, San Diego of Division of Biological Sciences for revising the English in the manuscript.References
- L. Li, N. Gao, Y. Deng, J. Yao and K. Zhang, Water Res., 2012, 46, 1233–1240 CrossRef CAS PubMed.
- F. Sun, H. Y. Pei, W. R. Hu and C. X. Ma, Chem. Eng. J., 2012, 193–194, 196–202 CrossRef CAS.
- M. Razali, Y. Zhao and M. Bruen, Sep. Purif. Technol., 2007, 55, 300–306 CrossRef CAS.
- D. I. Verrelli, D. R. Dixon and P. J. Scales, Water Res., 2010, 44, 1542–1552 CrossRef CAS PubMed.
- D. Caniani, S. Masi, I. M. Mancini and E. Trulli, Waste Manag., 2013, 33, 1461–1468 CrossRef CAS PubMed.
- X. Li, Y. Zhang, X. Zhao, N. Gao and T. Fu, Sep. Purif. Technol., 2015, 147, 125–131 CrossRef CAS.
- H. Y. Pei, C. X. Ma, W. R. Hu and F. Sun, Bioresour. Technol., 2014, 151, 314–322 CrossRef CAS PubMed.
- C. Ma, W. Hu, H. Pei, H. Xu and R. Pei, Colloids Surf., A, 2016, 490, 258–267 CrossRef CAS.
- G. Zhen, X. Lu, B. Wang, Y. Zhao, X. Chai, D. Niu, A. Zhao, Y. Li, Y. Song and X. Cao, Bioresour. Technol., 2012, 124, 29–36 CrossRef CAS PubMed.
- G. Zhen, X. Lu, Y. Li, Y. Zhao, B. Wang, Y. Song, X. Chai, D. Niu and X. Cao, Bioresour. Technol., 2012, 119, 7–14 CrossRef CAS PubMed.
- A. D. Eaton, E. W. Rice and R. B. Baird, Water Environment federation, in Standard methods for the examination of water and wastewater, American Public Health Association, American Water Work Association, Washington, D.C, 21st edn, 2005 Search PubMed.
- B. Frølund, T. Griebe and P. H. Nielsen, Appl. Microbiol. Biotechnol., 1995, 1995, 755–761 CrossRef.
- X. Zhang, P. L. Bishop and B. K. Kinkle, Water Sci. Technol., 1999, 39, 211–218 CAS.
- F. Qu, H. Liang, J. Tian, H. Yu, Z. Chen and G. Li, Desalination, 2012, 293, 30–37 CrossRef CAS.
- H. Rong, B. Gao, J. Li, B. Zhang, S. Sun, Y. Wang, Q. Yue and Q. Li, J. Colloid Interface Sci., 2013, 412, 39–45 CrossRef CAS PubMed.
- M. Ma, R. Liu, H. Liu, J. Qu and W. Jefferson, Sep. Purif. Technol., 2012, 86, 19–25 CrossRef CAS.
- H. Pei, H. Xu, H. Xiao, J. Sun, W. Hu, X. Li, C. Ma and Y. Jin, Colloids Surf., A, 2016, 499, 88–96 CrossRef CAS.
- Z. S. Chu, B. Yang, X. C. Jin, F. Yan, S. F. Zheng, Y. Pang and Q. R. Zeng, Environ. Sci., 2007, 28, 2695–2699 CAS.
- H. Wang, J. Qi, A. A. Keller, M. Zhu and F. Li, Colloids Surf., A, 2014, 450, 161–165 CrossRef CAS.
- F. Sun, W. Hu, H. Pei, X. Li, X. Xu and C. Ma, Sep. Purif. Technol., 2015, 150, 52–62 CrossRef CAS.
- D. Pantelic, Z. Svircev, J. Simeunovic, M. Vidovic and I. Trajkovic, Chemosphere, 2013, 91, 421–441 CrossRef CAS PubMed.
- M. Campinas and M. J. Rosa, Sep. Purif. Technol., 2010, 70, 345–353 CrossRef CAS.
- M. Campinas and M. J. Rosa, Sep. Purif. Technol., 2010, 71, 114–120 CrossRef CAS.
- F. Qu, H. Liang, Z. Wang, H. Wang, H. Yu and G. Li, Water Res., 2012, 46, 1490–1500 CrossRef CAS PubMed.
- R. K. Henderson, A. Baker, S. A. Parsons and B. Jefferson, Water Res., 2008, 42, 3435–3445 CrossRef CAS PubMed.
- E. Tipping and H. T. Carter, Sci. Total Environ., 2011, 409, 1550–1558 CrossRef CAS PubMed.
- C. D. Wu, X. J. Xu, J. L. Liang, Q. Wang, Q. Dong and W. L. Liang, Desalination, 2011, 279, 140–145 CrossRef CAS.
- Y. Liu, X. Li, Y. Yang and S. Liang, Desalination, 2015, 355, 75–82 CrossRef CAS.
- M. Ma, R. Liu, H. Liu and J. Qu, J. Hazard. Mater., 2012, 217–218, 279–285 CrossRef CAS PubMed.
- F. Qu, H. Liang, J. He, J. Ma, Z. Wang, H. Yu and G. Li, Water Res., 2012, 46, 2881–2890 CrossRef CAS PubMed.
- K. Li, F. Qu, H. Liang, S. Shao, Z. S. Han, H. Chang, X. Du and G. Li, Desalination, 2014, 336, 129–137 CrossRef CAS.
- T. Liu, Z. L. Chen, W. Z. Yu and S. J. You, Water Res., 2011, 45, 2111–2121 CrossRef CAS PubMed.
- Z. Wang, Z. Wu and S. Tang, Water Res., 2009, 43, 1533–1540 CrossRef CAS PubMed.
- X. Y. Li and S. F. Yang, Water Res., 2007, 41, 1022–1030 CrossRef CAS PubMed.
- G. P. Sheng, H. Q. Yu and X. Y. Li, Biotechnol. Adv., 2010, 28, 882–894 CrossRef CAS PubMed.
- J. R. Bordowitz and B. L. Montgomery, Sensors, 2010, 10, 6969–6979 CrossRef CAS PubMed.
- K. Listiarini, D. D. Sun and J. O. Leckie, J. Membr. Sci., 2009, 332, 56–62 CrossRef CAS.
- C. Y. Hu, S. L. Lo, C. L. Chang, F. L. Chen, Y. D. Wu and J. L. Ma, Sep. Purif. Technol., 2013, 104, 322–326 CrossRef CAS.
- S. Liang, L. Qu, F. Meng, X. Han and J. Zhang, J. Membr. Sci., 2013, 436, 186–194 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11989a |
| This journal is © The Royal Society of Chemistry 2016 |