Synthesis of stable CucoreAgshell&Ag particles for direct writing flexible paper-based electronics†
Wei Liab,
Yansong Wanga,
Mengmeng Wanga,
Wenjiang Lia,
Junjun Tanc,
Chen You*a and
Minfang Chen*ab
aSchool of Materials Science and Engineering, Tianjin University of Technology, Tianjin, 300384, China. E-mail: mfchentj@126.com; Tel: +86 022 60215845
bTianjin Key Lab for Photoelectric Materials & Devices, Tianjin, 300384, China
cSchool of Chemical and Materials and Engineering, Hubei University of Technology, Hubei 435003, China
First published on 22nd June 2016
Abstract
Highly conductive flexible paper-based patterns were drawn directly using a brush pen dipped in ink consisting of copper–silver core–shell with individual silver (CucoreAgshell&Ag) particles. Using a cost-effective and green method, the formation of these CucoreAgshell&Ag particles is first driven by a transmetalation reaction on the surface of copper nanoparticles between copper atoms and silver ions, and then excessive Ag ions were further reduced by the glucose to form individual Ag nanoparticles. Characterization of these particles by XRD, SEM and TGA confirm the CucoreAgshell&Ag structure and their stability towards oxidation. The conductivity of the bending pattern was experimentally tested with different bending angles, bending cycles and bending storage times. It was found that the silver shell with external excessive Ag NPs not only improves the packing density, but also enhancing the particle purity results in the high conductivity of the bending pattern. Sintered at a low temperature of 160 °C and after 3000 bending cycles and storage for 300 days, the linear resistivity of the pattern increased from ∼5 μΩ cm to ∼20 μΩ cm, 4 times higher than before the pattern was bent, which is acceptable for conductive patterns in practical applications. Thus, this approach represents a promising method for the formation of microelectrodes or electronic devices with good flexibility and conductivity.
1 Introduction
The fabrication of conductive patterns as microelectrodes/circuits on flexible substrates has grown to become a major topic in the manufacture of flexible and low-cost electronic devices.1–3 More approaches, such as photolithography technology and airbrush spraying and ink-jet printing, have been developed in recent years. However, these techniques usually require expensive equipment and generate some pollution and waste. Thus, there are many challenges to develop a simple, green technique for real manufacture. We have previously reported an inexpensive and environmentally friendly approach,4 in which a designed gel-ink pen loaded with conductive ink could be used to achieve the direct writing of electronics on paper. However, it is difficult to produce relatively large-area patterns such as microelectrodes.Conductive ink materials including carbon,5 conductive polymer,6 graphene,7 or metal–organic complex8 inks have been used in the formation of conductive patterns, but these materials typically exhibit low electrical conductivity and low resolution, poor chemical and thermal stability, and there are few reports on the preparation of these materials through a green method. Although noble metal NPs, such as gold and silver NPs, possess high conductivity and can be used as alternative materials,9–11 the high cost of these materials has limited their commercial applications. The use of the much cheaper copper with good conductivity is highly anticipated, but it is easily oxidized into either Cu2O or CuO under ambient conditions leading to an increase in the sintering temperature, greatly reducing the electrical conductivity of the pattern.12 Some anti-oxidation materials, such as silver and carbon, have been used as the shell of a Cu core.13,14 However, the thin-shell layer was easily broken when sintered at a high temperature, reducing the pattern’s conductivity.14 In addition, with the increase of the packing density of micrometer-sized metal patterns upon adding different size particles, the pattern’s electrical conductivity can be improved.15–17 So mixed metal-based inks,12,18 such as Ag and Cu, have been developed recently. However, this material, formed by the simple addition of particles, was easily oxidized after storage for a long time. Obviously, there is still a need to develop a new-type of conductive ink to solve the above mentioned problems. For the reported conductive and flexible patterns,19 the mechanical properties is an important factor for its practical application. However, there are very few reports on the patterns’ conductivity and microstructure when the patterns were bent or kept in bent shape for a long time.20,21
Here, we report a simple and green method to synthesise air-stable CucoreAgshell&Ag particles via mixed polyvinylpyrrolidone (PVP) and cetyltrimethylammonium bromide (CTAB) as capping agents, and made a corresponding conductive ink, which can be used to directly write on photo paper. Flexible paper-based patterns, sintered at a low temperature of 160 °C, were obtained. The conductivity, mechanical flexibility and microstructure of the patterns were investigated with different bending angles, number of bending cycles and bending storage time. This CucoreAgshell&Ag particle structure has an advantage in enhancing the flexibility of the pattern with minimal degradation of its electrical properties. Furthermore, using this simple method, flexible and conductive paper-based microelectrodes can be fabricated easily.
2 Experimental
2.1 Materials
Polyvinylpyrrolidone (PVP, Mw = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and cetyltrimethylammonium bromide (CTAB), supplied by Sinopharm Chemical Reagent Co. Ltd, Shanghai, China, were used in this investigation as capping agents. Sodium phosphinate monohydrate (NaH2·PO2·H2O) and glucose (C6H12O6), used as reducing agents, copper sulfate pentahydrate (CuSO4·5H2O), silver nitrate (AgNO3), ethanol, diethylene glycol (DEG), ethylene glycol (EG) and glycerol, supplied by Chemical Reagent Company, Tianjin, China, were used for preparing the CucoreAgshell&Ag NPs and CucoreAgshell&Ag particle ink. All these agents were used without further purification. Distilled water was used in all experiments.
000) and cetyltrimethylammonium bromide (CTAB), supplied by Sinopharm Chemical Reagent Co. Ltd, Shanghai, China, were used in this investigation as capping agents. Sodium phosphinate monohydrate (NaH2·PO2·H2O) and glucose (C6H12O6), used as reducing agents, copper sulfate pentahydrate (CuSO4·5H2O), silver nitrate (AgNO3), ethanol, diethylene glycol (DEG), ethylene glycol (EG) and glycerol, supplied by Chemical Reagent Company, Tianjin, China, were used for preparing the CucoreAgshell&Ag NPs and CucoreAgshell&Ag particle ink. All these agents were used without further purification. Distilled water was used in all experiments.
2.2 Preparation of CucoreAgshell&Ag particles and CucoreAgshell&Ag ink
Cu particles were firstly obtained by the reduction of CuSO4·5H2O using non-toxic NaH2·PO2·H2O as a reducing agent in the presence of PVP and CTAB as mixed capping agents. The typical procedure is as follows: 0.3 g of PVP, 0.3 g of CTAB and 0.5 g of CuSO4·5H2O were dissolved completely in 20 mL of EG to form solution A. The pH of the solution A was adjusted to 10 using NaOH. Then, NaH2·PO2·H2O was dissolved completely in 10 mL of distilled water to form the solution B. Finally, solution B was added to solution A, with stirring and heating at 120 °C for 1 h. After the color of the mixture solution changed to reddish brown, heating was stopped and the mixture was allowed to cool naturally to room temperature. Excess AgNO3 solution (102 g L−1, 10 mL) was dropped into the above solution, maintaining vigorous stirring. After 30 minutes, C6H12O6 solution (34 g L−1, 10 mL) was subsequently dropped into the solution. The color of the solution changed from gray black to light green. The precipitate was separated by centrifugation and washed with ethanol five times. Thus, the CucoreAgshell&Ag particles were obtained.To build a surface tension gradient between the solvents to acquire high quality CucoreAgshell&Ag ink, the obtained CucoreAgshell&Ag particles were redissolved into a mixture of water, ethanol, glycerol and ethylene glycol (their corresponding surface tensions being 72.8, 22.4, 63.3, 48.5 mN m−1, respectively) with corresponding vol% values of about 23.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.0
8.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30.5
30.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38.5. The CucoreAgshell&Ag NPs were dispersed by ultrasonic treatment to obtain 35 wt% CucoreAgshell&Ag ink and used for drawing flexible printed patterns.
38.5. The CucoreAgshell&Ag NPs were dispersed by ultrasonic treatment to obtain 35 wt% CucoreAgshell&Ag ink and used for drawing flexible printed patterns.
2.3 Preparation of CucoreAgshell&Ag patterns
The CucoreAgshell&Ag patterns were created by directly writing on a flexible photo paper using a brush pen dipped in CucoreAgshell&Ag ink. Firstly, the brush pen was washed and the photo paper was used as the flexible substrate without any other treatment. Patterns could then be easily drawn by the brush pen dipped in the CucoreAgshell&Ag ink on photo paper. Finally, the CucoreAgshell&Ag pattern (together with the photo paper) was sintered under an argon atmosphere at 160 °C for 2 h.2.4 Characterizations
The purity of the CucoreAgshell&Ag NPs was investigated by X-ray powder diffraction (XRD, D/Max 2500pc, Rigaku, Japan) with CuKa radiation (λ = 1.54059 Å). The morphology of the copper patterns after sintering was investigated by scanning electron microscopy (SEM, S-4800, Hitachi, Japan) with a voltage of 2.0 kV. A thermogravimetric study of the copper NPs was performed with an NETZSCH STA 499C thermal gravity analyzer. The particles were heated up to 600 °C at a ramping rate of 10 °C min−1 in air. The resistance of the printed patterns was measured by a digital multimeter (KJ9205, Ke Jie Co., Ltd., China).3 Results and discussion
3.1 Characteristics of CucoreAgshell&Ag particles
The synthesis scheme of the CucoreAgshell&Ag particles, including three steps, is shown in Fig. 1. Firstly, an aqueous dispersion of Cu NPs was prepared with the use of NaH2·PO2·H2O as reducing agent. At the second step, excess AgNO3 solution was dropped into the above solution, and with the addition of glucose solution as a reducing agent, Ag particles with residual Ag+ ions were obtained. Then, a transmetalation reaction on the surface of Cu particles was processed between the copper atoms and the residual Ag+ ions, leading to CucoreAgshell particles being formed. Finally, CucoreAgshell&Ag particles were obtained. It should be mentioned that the whole process was environmentally friendly without being too time or energy consuming. The reaction equations are as follows:| Cu2+ + H2PO2− + H2O → Cu↓ + H2PO3− + 2H+ | (I) |
| 2[Ag(NH3)2]2OH + C6H12O6 → 2Ag↓ + 3NH3↑ + H2O + CH2OH(CHOH4)COONH4 | (II) |
| Cu + 2Ag+ → Cu2+ + 2Ag↓ | (III) |
XRD patterns of the synthesized samples are shown in Fig. 2. As seen from line a in Fig. 2, without the silver nitrate solution being added to the solution, the diffraction peaks of the pure Cu particle sample presents three main characteristic peaks at 2θ = 43.3°, 50.4° and 74.08°, corresponding to the Miller indices (111), (200) and (220) of metallic Cu with a face-centered cubic (fcc) structure. While, as can be seen going from line a to line b, upon adding silver nitrate to the solution with the Cu NPs, we can see that the obtained particles not only show the crystalline copper characteristic peaks, but also show four characteristic peaks at 2θ = 38.2°, 44.2°, 64.4° and 77.4°, corresponding to the diffraction peaks of (111), (200), (220) and (311) of face-centered cubic (fcc) Ag, indicating the presence of both copper and silver with fcc crystal structures. Moreover, increasing the concentration of silver nitrate resulted in enhancing the diffraction intensity of the Ag phase (line c), and meanwhile, the crystalline copper characteristic peaks disappeared, indicating that the CucoreAgshell&Ag particles were observed.
 | ||
| Fig. 2 X-ray diffraction patterns for the obtained particles with different concentrations of AgNO3 from (a) 0 g L−1 to (b) 51 g L−1 and (c) 102 g L−1 in 10 mL. | ||
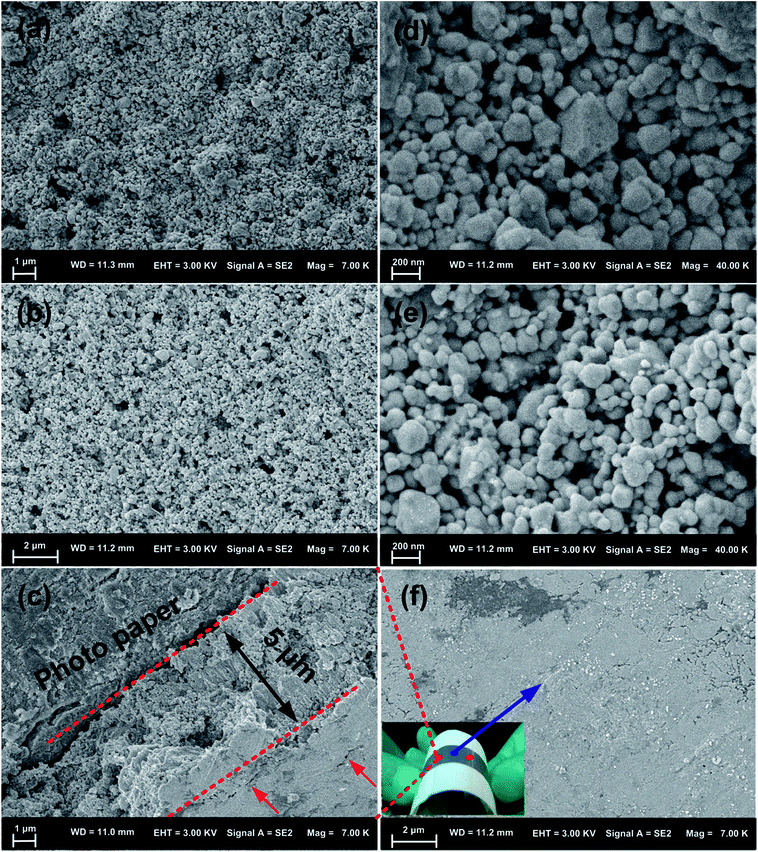
Fig. 3 shows SEM images of obtained particles and their particle size distributions. Many small particles among large particles were shown in Fig. 3a and d; the larger particle size is 1.1 ± 0.1 μm and the smaller particle size is 0.06 ± 0.04 μm. The number of small particles was not enough to be coated completely on the surface of the large particles, resulting in part CucoreAgshell particles obtained. This result was consistent with the XRD result from line b in Fig. 1. Upon increasing the silver nitrate concentration, more and more small particles with a size of 0.06 ± 0.04 μm appeared among the larger particles and the large particles became more and more large with a size of 1.7 ± 0.5 μm (Fig. 3b and e). Compared with the XRD result from line c in Fig. 1, this result indicates that CucoreAgshell&Ag particles were obtained. The core–shell structure of the CucoreAgshell&Ag particles was seen and the light silver shell was coated on the surface of the dark copper core, surrounded with small particles (ESI Fig. S1†). Fig. 2c shows the obtained spherical silver particles with a large distribution and a size of 0.06 ± 0.02 μm (Fig. 3f), which have a similar distribution tendency to the small particle distribution tendency in the inset images in Fig. 3d and e, indicating that the small particles were silver and the larger particles were copper.
Fig. 4 shows thermogravimetric curves of the CucoreAgshell&Ag particles obtained at temperatures ranging from room temperature to 700 °C in air. According to a previous report,22 the weight gain of the obtained particles occurs in four steps: (I) the first step from room temperature to 220 °C is a fast weight loss, attributed to the loss of residual alcohol and physisorbed water in the material and the weight loss was about 3%. (II) As the temperature increased between 220–400 °C, there was a low rate decomposition and oxidation stage as the organic capping layer gradually decomposed and the sample gained weight as the Ag NPs started to oxidize. In fact, the thermal decomposition of pure CTAB molecules was mainly performed at this temperature (Td = 200–300 °C). Because the gain weight is higher than the loss weight, the gain weight was partially offset, which was only about 0.9%. (III) The third step was a rapid oxidation stage at 400–550 °C, due to the weight loss from the accelerated decomposition of the residual organic molecules (mainly PVP, Td = 400–500 °C) and weight gain from the oxidation of silver or copper particles. The weight gain is much higher than the weight loss, so the weight gain increased greatly by about 2.3%. (IV) As the temperature increased ranging from 550 to 700 °C, weight gain or loss is not obvious, indicating that the oxidation process of silver or copper was completed. In theory, with all silver particles oxidized the weight gain was about 6.1% (molar ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 1
Ag = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), which was higher than the total virtual weight gain 3.1% in stages II and III; considering the partial offset of the weight loss of the organic capping molecules (<0.9%), more Ag particles were oxidized and the Ag shell was not completely oxidized, indicating the silver shell was a dense anti-oxidation layer for copper particles. These results suggested that the excess silver particles and silver shell, forming a dense layer, made the obtained pattern possess a high electrical conductivity even when the temperature was raised to 400 °C. Based on this result, more substrates needing a higher temperature can be used in more fields of application.
3), which was higher than the total virtual weight gain 3.1% in stages II and III; considering the partial offset of the weight loss of the organic capping molecules (<0.9%), more Ag particles were oxidized and the Ag shell was not completely oxidized, indicating the silver shell was a dense anti-oxidation layer for copper particles. These results suggested that the excess silver particles and silver shell, forming a dense layer, made the obtained pattern possess a high electrical conductivity even when the temperature was raised to 400 °C. Based on this result, more substrates needing a higher temperature can be used in more fields of application.
3.2 Conductivity of the CucoreAgshell&Ag patterns
To demonstrate the conductivity of the CucoreAgshell&Ag ink, an approximately 8 mm wide line was designed, as shown in Fig. 5. The width of the conductive line depends on the writing pressure and the tip size of the writing brush. This approach makes the formation of flexible electrodes/electronics easy and immediate. After the line was sintered at 160 °C for 2 h, a digital multimeter was used and the measured resistance of the conductive line was 1.8 Ω. According to the calculated formula for the electrical resistivity for the conductive patterns,18 the resistivity of the line was ∼5 μΩ cm, 3 times higher than the bulk Ag wire resistivity (1.6 μΩ cm), which is acceptable for portable applications. The inset shows a conductive wire connected to the positive and negative electrodes of a cell, and when the conductive wire is connected to the brushed conductive line, a lamp can immediately be turned on. The applied voltage was 3.0 V and the calculated current was ∼1.6 A, demonstrating that a conduction path between the particles is established and the conductive line is conductive. For the single Ag and Cu patterns (as shown in ESI Fig. S2†), the resistance was 114 Ω and 292 Ω, respectively, 64 and 162 times higher than the CucoreAgshell&Ag pattern, indicating that the structure of CucoreAgshell&Ag combined the particles’ purity and its packing density, leading to a lower resistivity. Thus, we can see that a larger pattern can be easily produced and other substrates can be used, such as Kapton (as shown in ESI Fig. S3†), which can be used as a microelectrode in many fields, such as triboelectronic nanogenerators and solar cells, etc. All of these results show that the brushing method with CucoreAgshell&Ag ink is an inexpensive, facile and promising approach to prepare conductive patterns for portable applications. | ||
| Fig. 5 Applications of paper-based CucoreAgshell&Ag conductive patterns drawn using a brush pen sintered at 160 °C for 2 h. | ||
The lowest resistivity of the pattern at a low temperature 160 °C is related to the state of the coated Cu particles. As illustrated in Fig. 6, in the form of CucoreAgshell, the silver shell transforms during heating into small particles, which are attached to the copper core surface, and above 250 °C, the Cu particles are no longer coated by a silver shell.14 However, in the form of CucoreAgshell&Ag, existing excess Ag particles resist the shell transformation into small particles, the Cu NPs are still coated by a silver shell at a high temperature, and this structure not only improved the high conductivity and increased the flexibility of the pattern after sintering at 250 °C for 2 h and 3000 bending cycles (ESI Fig. S3†). This explanation is consistent with the thermogravimetric curves of the CucoreAgshell&Ag particles.
 | ||
| Fig. 6 Schematic illustration of the growing silver crystallites forming shells as CucoreAgshell and CucoreAgshell&Ag, respectively. | ||
3.3 The application of the CucoreAgshell&Ag pattern
The mechanical flexibility of the CucoreAgshell&Ag patterns is an important factor for their application. Here, the flexibility of the pattern was tested experimentally and the bending pattern’s electrical resistance was measured. As shown in Fig. 7a, when the pattern with a bending angle kept at 90° was connected to a closed electric circuit, the ordinary small bulb was turned on immediately. As the bending angle increased to 180° and 330° (Fig. 7b and c), the small bulb was still turned on, but the light intensity gradually decreased. The corresponding resistance test also directly confirmed this result, and with the increase of the bending angle ranging from 90° to 180° and 330°, the pattern’s resistance gradually increased from 4.9 to 5.2 and 6.6 Ω (Fig. 7d–f), which is relatively low and acceptable for practical application. For the single Ag and Cu patterns, the resistance was greatly increased to 336 Ω and 686 Ω, respectively, 50 and 104 times higher than the CucoreAgshell&Ag patterns, and the ordinary small bulb was not turned on (ESI Fig. S2†). These results indicate that the external small silver NPs filled the interstices between the larger CucoreAgshell particles, which can benefit the bending patterns’ conductivity. This result confirmed that the CucoreAgshell&Ag patterns have an advantage in enhancing the flexibility with minimal degradation of their electrical properties.To further test the flexibility of the patterns, the bending pattern was kept at 330° and the resistance was measured as a function of storage time. The resistivity curve was shown in Fig. 8. When the storage time was increased from 0 to 100 days, owing to the blocked conduction path between the particles, the patterns’ resistivity gradually increased from ∼16.2 to 37.5 μΩ cm. While with the increase of the storage time to 300 days, the resistivity changed insignificantly, ∼38.5 μΩ cm, indicating that the blocked conduction path between the particles was stable, and this relatively low resistivity is also acceptable for practical application.
 | ||
| Fig. 8 The resistivity of the CucoreAgshell&Ag patterns with bending kept at 330° as a function of storage time; the inset shows the corresponding resistance of the bending pattern. | ||
Surface microstructures of the patterns sintered at 160 °C for 2 h before and after bending were observed by SEM (Fig. 9). It can be clearly seen that before bending, the surface of the pattern was composed of many excess particles (Fig. 9a), and the excessive smaller Ag NPs were able to fill the interstices between the larger CucoreAgshell particles, which was similar to the earlier reported single CucoreAgshell particles sintered at 250 °C,14 resulting in an increase in densification. These results indicate that the existence of excessive smaller Ag NPs leads to the pattern with a relatively low resistivity 5 μΩ cm (Fig. 5) at a lower temperature of 160 °C. After the bending pattern was kept at 330° for 300 days (Fig. 9b and e), the void spaces on the surface of the pattern became more obvious and from the cross-sectional area of the pattern (Fig. 9c and f), more small grooves and cracks were obvious, leading to the resistivity of the pattern increasing to 38.5 μΩ cm, which was about two orders of magnitude lower than that of the single Cu and Ag particles (the resistivity was ∼1.0 × 103 μΩ cm and 1.9 × 103 μΩ cm, respectively, ESI Fig. S2†). These results demonstrate that the conductive property of the pattern was affected by its microstructure, which is related to its particle densification. In theory, with the increase of the patterns’ bending angle, the distance between particles would gradually increase, especially the upwards surface of the pattern, leading to a decrease of the conductivity of the pattern. However, the CucoreAgshell&Ag patterned microelectrodes can withstand repeated bending and stretching to large levels of strain with partial degradation of their electrical properties. Thus, a quality CucoreAgshell&Ag pattern with low resistivity and high flexibility was obtained.
Fig. 10 shows the electrical resistivity of the CucoreAgshell&Ag pattern as a function of bending cycles with different measured conditions. The bending angle was kept at 330° (Fig. 7c). Increasing the number of bending cycles, measuring the sample whilst curved, the resistivity of the pattern greatly increases over less than 1000 cycles, and over 3000 cycles, the resistivity was 110 μΩ cm, which was higher than before bending. However, under linear measured conditions, the resistivity changed insignificantly from ∼20 μΩ cm, 3 times before bending over 3000 cycles, which indicates that the number of microcracks or void space was decreased and a conduction path was formed. We believe that the microcracks or void space was increased with the pattern being gradually bent and decreased with the existence of excessive small Ag NPs in the CucoreAgshell&Ag structures, resulting in the pattern possessing an acceptably lower resistivity with good flexibility. Thus, in the future, the conductivity of flexible patterns can be improved using stretchable conductive materials to avoid microcracks or void spaces.
 | ||
| Fig. 10 Electrical resistivity of the patterns as a function of bending cycles and measured before and after bending (named curve resistivity and linear resistivity), respectively. | ||
Surface microstructures of the patterns sintered at 160 °C for 2 h after 3000 bending cycles were observed by SEM (Fig. 11). It can be clearly seen that the void space on the surface of the pattern became more and more obvious (Fig. 11a), and from the cross-sectional area of the pattern (Fig. 9b), more small grooves and cracks were observed, leading to the resistivity of the pattern increasing to 110 μΩ cm. This is also confirmed by the ordinary small bulb operation of the bending pattern, displaying a weak light in the inset of Fig. 11a. For the single Ag or Cu patterns, a lamp can not be turned on under the same conditions, indicating that larger cracks were formed. These results demonstrate that the conductive property of the pattern was affected by its microstructure, related to its particle densification, and adding more small Ag NPs between the CucoreAgshell particles increases the densification, resulting in an increase in the patterns’ conductivity. Thus, the ink with CucoreAgshell&Ag particles is appropriate to be used as conductive materials, and the photo paper guarantees the flexibility and foldability of the substrate.
4 Conclusion
We have demonstrated a simple, green and effective method for the synthesis of 1.7 ± 0.5 μm CucoreAgshell particles with 0.06 ± 0.04 μm individual Ag nanoparticles. These particles were characterized by XRD, SEM and TGA to confirm the CucoreAgshell&Ag structure and their stability to oxidation. With these particles, the CucoreAgshell&Ag particles ink can be drawn directly on a flexible paper substrate, resulting in the formation of conductive patterns. The conductivity and flexibility of the pattern was experimentally tested with different bending angles, bending cycles and bending storage time. The Ag shell with external excessive Ag NPs not only improves the particle packing density but also enhances the purity of particles as well as the conductivity of the bending pattern. After bending over 3000 cycles and storage for 300 days, the linear resistivity of the CucoreAgshell&Ag patterns sintered at 160 °C increased from ∼5 μΩ cm to ∼20 μΩ cm, which was about two orders of magnitude lower than that of used single Cu and Ag particles. Thus, the CucoreAgshell&Ag structure has the advantage of enhancing the flexibility with minimal degradation of the electrical properties, which is acceptable for conductive patterns in practical applications which require flexibility.Acknowledgements
The authors acknowledge the financial support for this work from the National Nature Science Foundation of China (No. 51501129, 51371126), Tianjin "131" Innovative Talents (the third level in 2016), Science and Technology supporting program in Tianjin (No. 14ZCZDGX00007). Tianjin Key subject for Materials Physics and Chemistry, Key Laboratory of Display Materials & Photoelectric Devices.References
- T. H. Van Osch, J. Perelaer, A. W. de Laat and U. S. Schubert, Adv. Mater., 2008, 20, 343 CrossRef CAS.
- Y. Chen, J. Au, P. Kazlas, A. Ritenour, H. Gates and M. McCreary, Nature, 2003, 423, 136 CrossRef CAS PubMed.
- B. Y. Ahn, E. B. Duoss, M. J. Motala, X. Guo, S.-I. Park, Y. Xiong, J. Yoon, R. G. Nuzzo, J. A. Rogers and J. A. Lewis, Science, 2009, 323, 1590 CrossRef CAS PubMed.
- W. Li, M. Chen, J. Wei, W. Li and C. You, J. Nanopart. Res., 2013, 15, 1 Search PubMed.
- D.-Y. Cho, K. Eun, S.-H. Choa and H.-K. Kim, Carbon, 2014, 66, 530 CrossRef CAS.
- H. Sirringhaus, T. Kawase, R. H. Friend, T. Shimoda, M. Inbasekaran, W. Wu and E. P. Woo, Science, 2000, 290, 2123 CrossRef CAS PubMed.
- S. Pang, H. Yenny, X. Feng and M. Klaus, Adv. Mater., 2011, 23, 2779 CrossRef CAS PubMed.
- W. D. Yang, C. Y. Liu, Z. Y. Zhang, Y. Liu and S. D. Nie, J. Mater. Chem., 2012, 22, 23012 RSC.
- Y. L. Tai and Z. G. Yang, J. Mater. Chem., 2011, 21, 5938 RSC.
- S. R. Samarasinghe, I. Pastoriza-Santos, M. J. Edirisinghe, M. J. Reece and L. M. Liz-Marzán, Gold Bull., 2006, 39, 48 CrossRef.
- Z. Zhang, X. Zhang, Z. Xin, M. Deng, Y. Wen and Y. Song, Nanotechnology, 2011, 22, 425601 CrossRef PubMed.
- K. Woo, D. Kim, J. S. Kim, S. Lim and J. Moon, Langmuir, 2008, 25, 429 CrossRef PubMed.
- N. A. Luechinger, S. Loher, E. K. Athanassiou, R. N. Grass and W. J. Stark, Langmuir, 2007, 23, 3473 CrossRef CAS PubMed.
- P. Kic and R. Liška, J. Mater. Chem, 2009, 19, 3057 RSC.
- S. Solonin and L. Chernyshev, Powder Metall. Met. Ceram., 2006, 45, 226 CrossRef CAS.
- S. Grandjean, J. Absi and D. Smith, J. Eur. Ceram. Soc., 2006, 26, 2669 CrossRef CAS.
- I. Sumirat, Y. Ando and S. Shimamura, J. Porous Mater., 2006, 13, 439 CrossRef CAS.
- W. Li, W. Li, M. Wang, G. Liu and M. Chen, RSC Adv., 2016, 6, 10670 RSC.
- S. Jang, Y. Seo, J. Choi, T. Kim, J. Cho, S. Kim and D. Kim, Scr. Mater., 2010, 62, 258 CrossRef CAS.
- M. Aminuzzaman, A. Watanabe and T. Miyashita, J. Nanopart. Res., 2010, 12, 931 CrossRef CAS.
- A. C. Siegel, S. T. Phillips, M. D. Dickey, N. Lu, Z. Suo and G. M. Whitesides, Adv. Funct. Mater., 2010, 20, 28 CrossRef CAS.
- A. Yanase and H. Komiyama, Surf. Sci., 1991, 248, 11 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11965d |
| This journal is © The Royal Society of Chemistry 2016 |