Hirundigenin type C21 steroidal glycosides from Cynanchum stauntonii and their anti-inflammatory activity†
Chang-zhi Lai‡
a,
Hai-bin Liu‡b,
Jian-xin Liucd,
Qin Ouyange,
Shu-wen Panga,
Hua Zhouc,
Hai-yan Tiana,
Liang Liuc,
Xin-sheng Yao*a and
Jin-shan Tang *a
*a
aInstitutes of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou 510632, P. R. China. E-mail: tyaoxs@jnu.edu.cn; gztangjinshan@126.com; Fax: +86-20-85221559; Tel: +86-20-85220785
bGuangdong Lewwin Pharmaceutical Research Institute CO., Ltd, Guangzhou, P. R. China
cState Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, Avenida Wai Long, Taipa, Macau, P. R. China
dCollege of Pharmacy, Hunan University of Medicine, Huaihua 418000, P. R. China
eSchool of Pharmacy, The Third Military Medical University, Chongqing, 400038, PR China
First published on 17th June 2016
Abstract
Six new hirundigenin-type C21 steroidal glycosides, namely hirundigosides E–J (1–6), were obtained from the dried roots of Cynanchum stauntonii. Their chemical structures were elucidated by analyses of HR ESI-TOF MS, 1D, 2D-NMR, and X-ray crystallographic methods together with acidic hydrolysis. Interestingly, they exist in nature as epimers owing to the presence of 14-hemiketal hydroxyl group. Moreover, it is striking that only one set of resonance signals (14β-OH epimer) appeared in the 1H and 13C NMR spectra of compounds 1–6 when measured in CDCl3, while two sets of resonance signals (14α- and 14β-OH epimers) were observed when measured in pyridine-d5. It may ascribe to the favorable formation of intramolecular hydrogen bond of aglycone due to the proximate distance of hydroxyl group and oxygen atoms (1.86 nm) in 14β-OH epimer in CHCl3. The difference of potential energy (ΔG) of 14α-OH epimer to 14β-OH epimer is about 0.73 kcal mol−1 for compound 1 in CHCl3, while it is unfavorable for the formation of intramolecular hydrogen bond in pyridine and the G values of them are almost equal in 1. Also, CDCl3 is neither protic solvent nor Lewis acid and it can't induce the isomerization of hemiketal hydroxyl group in hirundigosides, while pyridine is Lewis base and it promotes the isomerization of hirundigosides. In addition, secondary anhydrohirundigenin-type glycosides and anhydrohirundigenin were obtained by mild acidic hydrolysis and their anti-inflammatory activities were also discussed.
The dried roots of Cynanchum stauntonii (Decne.) Schltr.ex Lévl., together with the other species of the same genus, C. glaucescens (Decne.) Hand.-Mazz., are recorded in the Chinese Pharmacopoeia (ChP) as “Bai-Qian”, and have been used as antitussive and expectorants in China for a long time.1 Chemical investigation revealed that they contain characteristic C21 steroidal glycosides, especially with the aberrant 13,14:14,15-disecopregnane-type skeleton or 14,15-secopregnane-type skeleton, which exhibited antitumor activity and inhibitory effects on Na+/K+-ATPase and alpha virus-like RNA viruses.2–4
In our search for anti-inflammatory constituents from the roots of C. stauntonii,5 six new hirundigenin-type C21 steroidal glycosides, namely hirundigosides E–J (1–6), were obtained from the dried roots of C. stauntonii. Herein, we described the isolation and structural elucidation of the new compounds. Meanwhile, we provided the evidence that they existed in nature as epimers owing to the presence of 14-hemiketal hydroxyl group by UPLC-MS/MS and chemical derivatization. Also, the abnormal behavior of their NMR spectrum in different deuterated solvents was discussed by computational calculation. In addition, secondary anhydrohirundigenin-type glycosides and anhydrohirundigenin were obtained by mild acidic hydrolysis of compounds 2 and 6 and their anti-inflammatory activities were also discussed.
Results and discussion
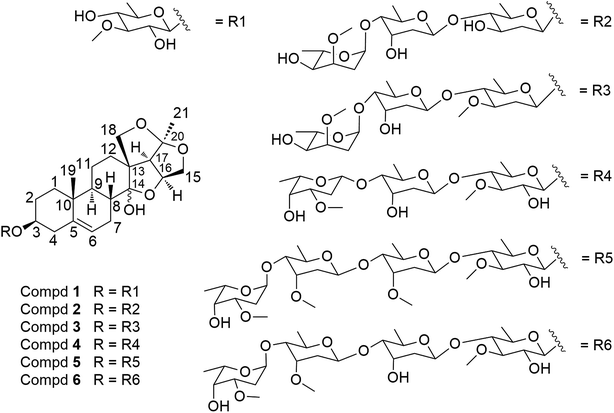
The 95% (v/v) aqueous EtOH extract of the roots of C. stauntonii was chromatographed over an open macroporous resin Diaion HP 20 column eluted with gradient EtOH–H2O to obtained 4 fractions (A–D). The fraction D (93.0 g, 95% EtOH) was repeatedly subjected to silica gel, Sephadex LH-20, and RP-C18 column chromatography (CC) and semipreparative RP-HPLC to afford 6 new 14,15-secopregnane steroidal glycosides (1–6) (Fig. 1).Compounds 1–6 showed positive reactions to Libermann–Burchard and Keller–Kiliani reagents, suggesting that they were likely steroidal glycosides with 2-deoxysugar units in the structures.
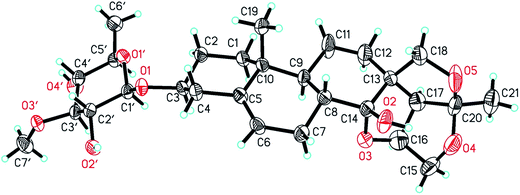
Hirundigoside E (1), [α]27D −15.2 (MeOH, c = 0.5), was obtained as colorless needle crystals (CHCl3/MeOH). Its molecular formula, C28H42O9, was deduced from the positive-ion HR ESI-TOF MS (m/z 545.2732 [M + Na]+, calcd for C28H42O9Na, 545.2727), indicating eight degrees of unsaturation. The IR spectrum showed absorption bands for hydroxyl (3441 cm−1) and olefinic (1455, 1165 cm−1) functionalities. The 1H NMR spectrum of aglycone portion exhibited two tertiary methyl protons at δH 0.91 (3H, s, H-19) and 1.44 (3H, s, H-21), one olefinic proton signal at δH 5.40 (1H, d, J = 4.9 Hz, H-6), two oxygen-substituted methine protons at δH 3.49 (1H, m, H-3) and 4.69 (1H, q, J = 3.5 Hz, H-16), two methylene protons at δH 3.72 (1H, m, H-15α)/4.16 (1H, d, J = 10.9, H-15β) and 3.73 (1H, m, H-18α)/4.48 (1H, d, J = 9.0, H-18β) (Table 1). The 13C NMR spectrum showed two oxygenated tertiary carbon signals at δC 108.3 (C-14) and 118.6 (C-20), two oxygenated methylene carbon signals at δC 75.3 (C-15) and 77.9 (C-18), and two olefinic carbons at δC 139.1 (C-5) and 122.7 (C-6) (Table 3). Comparison of 1H and 13C NMR data with that of hirundigoside D,6 a known steroidal glycoside isolated from the roots of Vincetoxicum hirundinaria, revealed that compound 1 contained the aglycone of hirundigenin. An anomeric proton and carbon signals at δH 4.29 (1H, d, J = 7.7 Hz)/δC 101.1 in the 1H and 13C NMR spectra, along with the presence of one secondary methyl proton signals at δH 1.26 (3H, d, J = 6.1 Hz), suggested that 1 contained one 6-deoxysugar with β glycosidic linkage. The sugar moiety was defined as thevetose by its 13C resonance data, which matched well with that of anhydrohirundigenin monothevetoside.7 The HMBC correlation from δH 4.29 (H-1′) to δC 79.1 (C-3) attached the sugar moiety at C-3 position of the aglycone. The relative configuration of 1 was established by ROESY experiment (Fig. 2). The ROESY correlation from CH3-19 to H-8 indicated that H-8 was β-oriented; while the correlation from 14-OH to 19-CH3, 15β-H, and 18β-H suggested the relative orientation of 14-OH was β. The relative configuration of compound 1 was confirmed by X-ray diffraction analysis (Fig. 3). Mild acid hydrolysis of 1 followed by column chromatography yielded thevetose. The specific rotation value of thevetose ([α]27D + 19.9) was obtained from an aqueous solution after 24 h equilibration. The D-configuration of thevetose was defined by comparison of the experimental and reported specific rotation values.5,8 Thus, the structure of 1 was defined as hirundigenin 3-O-β-D-thevetopyranoside named hirundigoside E.
| Position | 1b | 2a | 3b | 4b | 5b | 6a |
|---|---|---|---|---|---|---|
| a Spectra in 600 MHz.b Spectra in 400 MHz. | ||||||
| 1 | 1.79, m/1.00, td(13.5, 3.5) | 1.80, m/0.98, td(13.5, 3.5) | 1.79, m/1.00, td(13.9, 3.6) | 1.82, m/1.00, td(13.7, 3.5) | 1.79, m/1.00, td(13.9, 3.6) | 1.80, m/0.98, td(13.7, 3.3) |
| 2 | 1.88, m/1.57, m | 1.87, m/1.54, m | 1.88, m/1.55, m | 1.89, m/1.58, m | 1.88, m/1.55, m | 1.88, m/1.55, m |
| 3 | 3.49, m | 3.48, m | 3.49, m | 3.49, m | 3.49, m | 3.48, m |
| 4 | 2.35, m/2.13, m | 2.30, m/2.14, m | 2.30, m/2.15, m | 2.35, m/2.16, m | 2.34, m/2.17, m | 2.34, m/2.17, m |
| 5 | ||||||
| 6 | 5.40, d(4.9) | 5.38, d(4.4) | 5.39, d(4.4) | 5.41, d(5.2) | 5.40, d(5.3) | 5.39, d(5.3) |
| 7 | 2.29, m/2.13, m | 2.30, m/2.13, m | 2.30, m/2.13, m | 2.31, m/2.13, m | 2.30, m/2.13, m | 2.30, m/2.13, m |
| 8 | 1.80, m | 1.79, m | 1.80, m | 2.09, m | 2.10, m | 2.07, m |
| 9 | 1.29, m | 1.30, m | 1.30, m | 1.30, m | 1.30, m | 1.28, m |
| 10 | ||||||
| 11 | 1.46, m/1.22, m | 1.43, m/1.25, m | 1.46, m/1.24, m | 1.47, m/1.23, m | 1.46, m/1.24, m | 1.46, m/1.24, m |
| 12 | 1.66, m/1.48, m | 1.67, m/1.45, m | 1.68, m/1.47, m | 1.68, m/1.47, m | 1.68, m/1.47, m | 1.66, m/1.47, m |
| 13 | ||||||
| 14-OH | 5.15, s | 5.15, s | 5.15, s | 5.15, s | 5.15, s | 5.15, s |
| 15 | 4.16, d(10.9)/3.72, m | 4.15, d(10.9)/3.72, m | 4.16, d(10.9)/3.71, m | 4.17, d(11.0)/3.73, dd(11.0, 3.8) | 4.16, d(10.8)/3.71, m | 4.16, d(10.8)/3.71, m |
| 16 | 4.69, q(3.5) | 4.69, q(3.6) | 4.70, q(3.6) | 4.70, m | 4.70, m | 4.70, m |
| 17 | 2.84, d(7.2) | 2.83, d(7.2) | 2.84, d(7.1) | 2.84, d(7.2) | 2.84, d(7.1) | 2.84, d(7.1) |
| 18 | 4.48, d(9.0)/3.73, m | 4.47, d(9.0)/3.74, m | 4.48, d(9.2)/3.74, m | 4.49, d(9.0)/3.75, m | 4.48, d(9.0)/3.74, m | 4.47, d(9.0)/3.73, m |
| 19 | 0.91, s | 0.90, s | 0.91, s | 0.92, s | 0.91, s | 0.91, s |
| 20 | ||||||
| 21 | 1.44, s | 1.43, s | 1.44, s | 1.45, s | 1.44, s | 1.43, s |
| Position | 1b | 2a | 3b | 4b | 5b | 6a |
|---|---|---|---|---|---|---|
| a Spectra in 600 MHz.b Spectra in 400 MHz. | ||||||
| The | Can | Ole | The | The | The | |
| 1′ | 4.29, d(7.7) | 4.55, d(9.6) | 4.50, dd(9.8, 1.8) | 4.29, d(7.8) | 4.28, d(7.8) | 4.27, d(7.9) |
| 2′ | 3.36, m | 2.30, m/1.54, m | 2.21, m/1.49, m | 3.37, m | 3.33, m | 3.33, m |
| 3′ | 3.07, m | 3.54, m | 3.32, m | 3.19, m | 3.19, m | 3.17, m |
| 4′ | 3.14, m | 2.92, t(8.8) | 3.15, t(8.8) | 3.25, m | 3.25, m | 3.16, m |
| 5′ | 3.32, m | 3.26, m | 3.25, m | 3.30, m | 3.30, m | 3.30, m |
| 6′ | 1.26, d(6.1) | 1.26, d(6.6) | 1.26, d(6.1) | 1.22, d(6.2) | 1.23, d(5.9) | 1.24, d(6.6) |
| 3′-OCH3 | 3.60, s | 3.58, s | 3.57, s | 3.56, s | ||
| Digt | Digt | Digt | Cym | Digt | ||
| 1′′ | 4.77, d(9.6) | 4.96, dd(9.7, 1.9) | 4.92, dd(9.7, 1.6) | 4.69, d(8.5) | 4.92, d(9.5) | |
| 2′′ | 2.14, m/1.70, m | 2.09, m/1.66, m | 2.09, m/1.65, m | 2.20, m/1.59, m | 2.07, m/1.63, m | |
| 3′′ | 4.03, d(2.5) | 4.02, d(3.2) | 4.19, d(2.8) | 3.74, m | 4.18, d(2.9) | |
| 4′′ | 3.21, m | 3.20, m | 3.17, m | 3.20, m | 3.17, m | |
| 5′′ | 3.84, m | 3.73, m | 3.78, m | 3.81, m | 3.74, m | |
| 6′′ | 1.22, d(5.6) | 1.21, d(6.1) | 1.24, d(6.1) | 1.19, d(6.0) | 1.25, d(6.6) | |
| 3′′-OCH3 | 3.39, s | |||||
| Cym | Cym | Dign | Cym | Cym | ||
| 1′′′ | 4.85, d(3.6) | 4.87, d(3.9) | 4.70, d(7.9) | 4.85, dd(9.2, 1.3) | 4.75, d(9.3) | |
| 2′′′ | 2.26, m/1.67, m | 2.33, m/1.71, m | 2.21, m/1.58 | 2.20, m/1.51, m | 2.18, m/1.57, m | |
| 3′′′ | 3.59, m | 3.59, m | 3.60, m | 3.34, m | 3.71, m | |
| 4′′′ | 3.21, m | 3.21, m | 3.15, m | 3.18, m | 3.16, m | |
| 5′′′ | 3.82, m | 3.81, m | 3.57, m | 3.76, m | 3.71, m | |
| 6′′′ | 1.21, d(6.0) | 1.22, d(6.2) | 1.23, d(6.3) | 1.18, d(6.0) | 1.18, d(6.6) | |
| 3′′′-OCH3 | 3.36, s | 3.36, s | 3.41, s | 3.37, s | 3.37, s | |
| Dign | Dign | |||||
| 1′′′ | 4.95, d(3.3) | 4.94, d(2.8) | ||||
| 2′′′ | 1.92, m/1.80, m | 1.86, m/1.55, m | ||||
| 3′′′ | 3.62, m | 3.33, m | ||||
| 4′′′ | 3.81, m | 3.74, m | ||||
| 5′′′ | 3.95, m | 3.94, m | ||||
| 6′′′ | 1.26, d(6.6) | 1.22, d(6.2) | ||||
| 3′′′-OCH3 | 3.35, s | 3.34, s | ||||
| Position | 1b | 2a | 3b | 4b | 5b | 6a |
|---|---|---|---|---|---|---|
| a Spectra in 150 MHz.b Spectra in 100 MHz. | ||||||
| 1 | 36.9 | 36.9 | 36.9 | 36.9 | 36.9 | 36.9 |
| 2 | 29.4 | 29.4 | 29.4 | 29.5 | 29.4 | 29.4 |
| 3 | 79.1 | 78.1 | 78.1 | 79.0 | 78.9 | 78.9 |
| 4 | 38.6 | 38.5 | 38.6 | 38.6 | 38.6 | 38.6 |
| 5 | 139.1 | 139.5 | 139.5 | 139.3 | 139.2 | 139.2 |
| 6 | 122.7 | 122.3 | 122.4 | 122.6 | 122.6 | 122.6 |
| 7 | 26.2 | 26.2 | 26.2 | 26.3 | 26.2 | 26.2 |
| 8 | 37.5 | 37.5 | 37.5 | 37.5 | 37.5 | 37.5 |
| 9 | 44.1 | 44.1 | 44.2 | 44.2 | 44.1 | 44.1 |
| 10 | 38.1 | 38.1 | 38.1 | 38.1 | 38.1 | 38.1 |
| 11 | 21.0 | 20.9 | 21.0 | 21.0 | 21.0 | 20.9 |
| 12 | 32.5 | 32.5 | 32.6 | 32.6 | 32.5 | 32.5 |
| 13 | 59.7 | 59.7 | 59.7 | 59.7 | 59.7 | 59.7 |
| 14 | 108.3 | 108.3 | 108.3 | 108.3 | 108.3 | 108.2 |
| 15 | 75.3 | 75.3 | 75.3 | 75.4 | 75.3 | 75.3 |
| 16 | 80.7 | 80.7 | 80.7 | 80.8 | 80.7 | 80.7 |
| 17 | 65.8 | 65.8 | 65.8 | 65.8 | 65.8 | 65.8 |
| 18 | 77.9 | 77.9 | 78.0 | 78.0 | 77.9 | 77.9 |
| 19 | 18.3 | 18.3 | 18.5 | 18.3 | 18.3 | 18.3 |
| 20 | 118.6 | 118.6 | 118.6 | 118.7 | 118.6 | 118.6 |
| 21 | 22.5 | 22.5 | 22.5 | 22.5 | 22.5 | 22.5 |
| The | Can | Ole | The | The | The | |
| 1′ | 101.1 | 97.9 | 97.8 | 100.9 | 100.9 | 100.9 |
| 2′ | 74.4 | 38.9 | 36.8 | 73.7 | 73.8 | 73.7 |
| 3′ | 85.6 | 69.9 | 79.3 | 84.3 | 84.3 | 84.4 |
| 4′ | 74.8 | 88.5 | 82.8 | 82.2 | 82.6 | 82.5 |
| 5′ | 71.7 | 70.5 | 71.3 | 71.5 | 71.5 | 71.4 |
| 6′ | 17.9 | 17.9 | 18.1 | 18.4 | 18.5 | 18.4 |
| 3′-OCH3 | 60.7 | 56.5 | 59.9 | 60.0 | 59.9 | |
| Digt | Digt | Digt | Cym | Digt | ||
| 1′′ | 99.4 | 98.8 | 98.9 | 99.6 | 98.3 | |
| 2′′ | 36.8 | 37.2 | 37.2 | 35.5 | 37.2 | |
| 3′′ | 67.5 | 67.8 | 66.7 | 77.1 | 66.7 | |
| 4′′ | 79.7 | 79.7 | 82.6 | 81.9 | 82.1 | |
| 5′′ | 69.0 | 68.8 | 68.4 | 68.9 | 69.1 | |
| 6′′ | 17.9 | 18.3 | 18.4 | 18.2 | 18.2 | |
| 3′′-OCH3 | 58.1 | |||||
| Cym | Cym | Dign | Cym | Cym | ||
| 1′′′ | 97.8 | 97.6 | 98.3 | 98.7 | 98.7 | |
| 2′′′ | 31.1 | 31.1 | 33.9 | 34.0 | 34.2 | |
| 3′′′ | 75.3 | 75.2 | 77.5 | 77.2 | 77.0 | |
| 4′′′ | 72.1 | 72.2 | 72.4 | 81.8 | 81.7 | |
| 5′′′ | 66.0 | 65.9 | 71.2 | 67.7 | 67.7 | |
| 6′′′ | 18.0 | 18.3 | 18.4 | 18.3 | 18.3 | |
| 3′′′-OCH3 | 56.6 | 56.7 | 57.6 | 57.0 | 57.2 | |
| Dign | Dign | |||||
| 1′′′ | 100.7 | 100.7 | ||||
| 2′′′ | 30.0 | 29.9 | ||||
| 3′′′ | 74.5 | 74.5 | ||||
| 4′′′ | 68.9 | 68.4 | ||||
| 5′′′ | 66.2 | 66.3 | ||||
| 6′′′ | 17.2 | 17.1 | ||||
| 3′′′-OCH3 | 55.7 | 55.6 | ||||
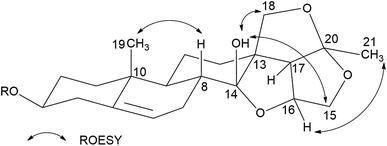 | ||
| Fig. 2 Key ROESY correlations of the aglycone of hirundigenin type C21 steroidal glycosides in CDCl3. | ||
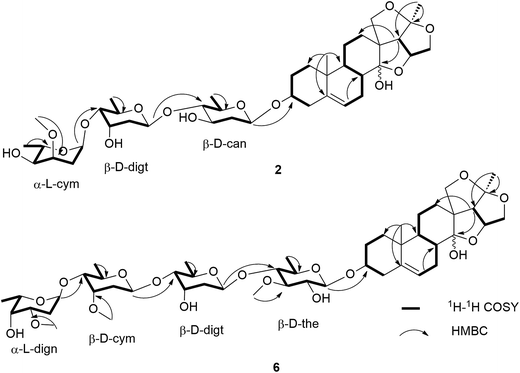
Hirundigoside F (2) was obtained as colorless amorphous powder. Its molecular formula was determined as C40H62O14 based on the positive HR ESI-TOF MS (m/z 789.4027 [M + Na]+). The IR spectrum showed the absorption bands for hydroxyl (3441 cm−1) and olefinic (1454 cm−1) groups. The 1H and 13C NMR data were assigned by analyses of 1H–1H COSY, HSQC and HMBC spectra (Tables 1 and 3). By comparing its NMR data with that of 1, compound 2 had the same aglycone as that of 1. Three anomeric carbon signals at δC 99.4, 97.9, and 97.8 in the 13C NMR spectrum were observed, corresponding to the anomeric proton resonances at δH 4.55 (1H, d, J = 9.6 Hz), 4.77 (1H, d, J = 9.6 Hz), and 4.85 (1H, d, J = 3.6 Hz) in the 1H NMR spectrum, respectively, suggesting that it contained three sugar units in the structure. The presence of three secondary methyl proton signals at δH 1.21 (3H, d, J = 6.0 Hz), 1.22 (3H, d, J = 5.6 Hz), and 1.26 (3H, d, J = 6.6 Hz) revealed that all of them were 6-deoxysugars. Analyses of 1H–1H COSY, HSQC and HMBC spectra enabled the identification of the three sugar units as cannarose, digitoxose, and cymarose (Fig. 4). The HMBC correlations from δH 4.85 (H-1′′′) to δC 79.7 (C-4′′) and from δH 4.77 (H-1′′) to δC 88.5 (C-4′) suggested that they were 1 → 4 linkages. The HMBC correlation from δH 4.55 (H-1′) to δC 78.1 (C-3) linked the sugar chain to C-3 position of the aglycone. The 13C NMR spectroscopic data of the sugar chain for 2 was quite similar with that of glaucogenin C 3-O-α-L-cymaropyranosyl-(1 → 4)-β-D-digitoxopyranosyl-(1 → 4)-β-D-cannaropyranoside,9 which also supported above deduction. The monosugars were obtained by mild acidic hydrolysis of 2 and silica gel column chromatography purification. Their absolute configurations were identified to be L-cymarose, D-digitose, and D-cannose according to their specific rotation values.5,10–12 The splitting patterns of anomeric proton signals indicated that the L-cymaropyranose was α-linkage and the other two sugars were β-linkages. Therefore, 2 was identified to be hirundigenin 3-O-α-L-cymaropyranosyl-(1 → 4)-β-D-digitoxopyranosyl-(1 → 4)-β-D-canaropyranoside named hirundigoside F.
Hirundigoside G (3) was obtained as colorless amorphous powder. It had the molecular formula of C41H64O14 by its pseudomolecular ion at m/z 803.4194 [M + Na]+ in the positive HR ESI-TOF MS. By comparing its NMR data with that of 1 (Tables 1 and 3), compound 3 was deduced to have the same aglycone as 1 and the difference between them lay in the sugar chains. The 1H NMR spectrum of 3 showed the presence of three anomeric proton signals at δH 4.50 (1H, dd, J = 9.8, 1.8 Hz), 4.87 (1H, d, J = 3.9 Hz), and 4.96 (1H, dd, J = 9.7, 1.9 Hz) and three secondary methyl proton signals at δH 1.21 (3H, d, J = 6.1 Hz), 1.22 (3H, d, J = 6.2 Hz), and 1.26 (3H, d, J = 6.1 Hz), which revealed that it was a triglycoside with 6-deoxysugars. The sugar moieties were determined to be cymarose, digitoxose and oleandrose by analyzing their 13C resonance data (Table 3). Furthermore, comparison of its NMR data with that of stauntoside K13 suggested that compound 3 had the same sugar chain in the structures, which supported the above deduction. The 1 → 4 connections of the three sugar units were determined by HMBC correlations from δH 4.87 (H-1′′′) to δC 79.7 (C-4′′) and from δH 4.96 (H-1′′) to δC 82.8 (C-4′). The HMBC correlation from δH 4.50 (H-1′) to δC 78.1 (C-3) enabled the location of the sugar chain at C-3 of the aglycone. The splitting patterns of anomeric proton signals indicated that the cymaropyranose was α-linkage and the other two sugars were β-linkages. The L configuration of cymarose and D configurations of digitoxose and oleandrose were defined according to their specific rotation values after mild acidic hydrolysis of 3 and subsequent purification.5,8,11,12 Thus, compound 3 was identified as hirundigenin 3-O-α-L-cymaropyranosyl-(1 → 4)-β-D-digitoxopyranosyl-(1 → 4)-β-D-oleandropyranoside named hirundigoside G.
Hirundigoside H (4) was obtained as colorless amorphous powder. It had the molecular formula of C41H64O15 based on the HR ESI-TOF MS (m/z 803.4194 [M + Na]+). By comparing its NMR data with that of 1 (Tables 1 and 3), compound 4 was deduced to share the same aglycone as 1. Three glycosyl units should be present in the structure of 4 according to three anomeric proton signals at δH 4.29 (1H, d, J = 7.8 Hz), 4.70 (1H, d, J = 7.9 Hz), and 4.92 (1H, dd, J = 9.7, 1.6 Hz) in its 1H NMR spectrum. The presence of three secondary methyl proton signals at δH 1.22 (3H, d, J = 6.2 Hz), 1.23 (3H, d, J = 6.3 Hz), and 1.24 (3H, d, J = 6.1 Hz) revealed that all of them were 6-deoxysugars. Comparison of the 1H and 13C NMR data with that of stauntoside I13 suggested that they had the same sugar chain in the structure, which was confirmed by analyses of 1H–1H COSY, HSQC and HMBC spectra. The HMBC correlation from δH 4.29 (H-1′) to δC 79.0 (C-3) enabled the location of sugar chain at C-3 of the aglycone. The L-configuration of diginose and D-configurations of digitoxose and oleandrose were defined according to their specific rotation values after mild acidic hydrolysis of 4 and subsequent purification.5,8,11,12 The splitting patterns of anomeric proton signals indicated that the three sugars were β-linkages. Thus, 4 was identified to be hirundigenin 3-O-β-L-diginopyranosyl-(1 → 4)-β-D-digitoxopyranosyl-(1 → 4)-β-D-oleandropyranoside named hirundigoside H.
Hirundigoside I (5) was obtained as colorless amorphous powder. The positive mode HR ESI-TOF MS showed a pseudomolecular ion at m/z 977.5084 [M + Na]+, revealing its molecular formula to be C49H78O18. By comparing its NMR data with that of 1 (Tables 1 and 3), compound 5 was deduced to have the same aglycone as 1. The 1H NMR spectrum of 5 showed the presence of four anomeric proton signals at δH 4.28 (1H, d, J = 7.8 Hz), 4.69 (1H, d, J = 8.5 Hz), 4.85 (1H, dd, J = 9.2, 1.3 Hz), and 4.95 (1H, d, J = 3.3 Hz) and four secondary methyl proton signals at δH 1.18 (3H, d, J = 6.0 Hz), 1.19 (3H, d, J = 6.0 Hz), 1.23 (3H, d, J = 5.9 Hz), and 1.26 (3H, d, J = 6.6 Hz), which revealed that it was a tetraglycoside with 6-deoxysugars. Assignments of 1H and 13C NMR data for each sugar unit were achieved by analyses of 1H–1H COSY, HSQC, HMBC and HSQC-TOCSY spectra. The sugar moieties in 5 were determined to be cymarose (×2), diginose, and thevetose by comparing its 13C NMR data with those of stauntoside E and stauntoside H.13 The 1 → 4 connections of the four sugar units were determined by the HMBC correlations from δH 4.95 (H-1′′′) to δC 81.8 (C-4′′′), from δH 4.85 (H-1′′′) to δC 82.8 (C-4′′), and from δH 4.69 (H-1′′) to δC 81.9 (C-4′). The HMBC correlation from δH 4.28 (H-1′) to δC 78.9 (C-3) enabled the location of the sugar chain at C-3 of the aglycone. The splitting patterns of anomeric proton signals indicated that the diginopyranose was α-linkage and the other three sugars were β-linkages. The D-cymarose, D-thevetose, and L-diginose were determined by their specific rotation values after mild acidic hydrolysis of 5 and subsequent purification.5,8,11 Thus, compound 5 was identified to be hirundigenin 3-O-α-L-diginopyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D-thevetopyranoside named hirundigoside I.
Hirundigoside J (6) was obtained as colorless amorphous powder. The molecular formula was deduced as C48H76O18 by the pseudomolecular ion at m/z 963.4912 [M + Na]+ in the positive mode HR ESI-TOF MS. Compounds 6 and 1 possessed the identical aglycones according to their 1H and 13C NMR data (Tables 1 and 3). The 1H and 13C NMR spectra of 6 showed four anomeric proton and carbon signals at δH 4.27 (d, J = 7.9 Hz)/δC 100.9, 4.94 (d, J = 2.8 Hz)/100.7, 4.75 (d, J = 9.3 Hz)/98.7, and 4.92 (d, J = 9.5 Hz)/98.3, indicating that it was a tetraglycoside. The presence of four secondary methyl proton signals at δH 1.18 (3H, d, J = 6.6 Hz), 1.22 (3H, d, J = 6.2 Hz), 1.24 (3H, d, J = 6.6 Hz), and 1.25 (3H, d, J = 6.6 Hz) revealed that all of them were 6-deoxysugars. Analyses of the 1H–1H COSY, HSQC, HMBC and HSQC-TOCSY spectra enabled the identification of these four sugar units as thevetose, digitoxose, cymarose, and diginose (Fig. 4). The 13C NMR spectroscopic data of the sugar chain in 6 was quite similar with that of stauntoside A,14 which also supported the above deduction. The 1 → 4 connections of the four sugar units were determined by HMBC correlations from δH 4.94 (H-1′′′) to δC 81.7 (C-4′′′), from δH 4.75 (H-1′′′) to δC 82.1 (C-4′′), and from δH 4.92 (H-1′′) to δC 82.5 (C-4′). The HMBC correlation from δH 4.27 (H-1′) to δC 78.9 (C-3) enabled the location of the sugar chain at C-3 of the aglycone. The absolute configurations of D-cymarose, D-thevetose, D-digitoxose, and L-diginose were determined by their specific rotation values after mild acidic hydrolysis of 6 and subsequent purification.5,8,11 The splitting patterns of anomeric proton signals indicated that the L-diginopyranose was α-linkage and the other three sugars were β-linkages. Therefore, compound 6 was identified to be hirundigenin 3-O-α-L-diginopyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D-digitoxopyranosyl-(1 → 4)-β-D-thevetopyranoside named hirundigoside J.
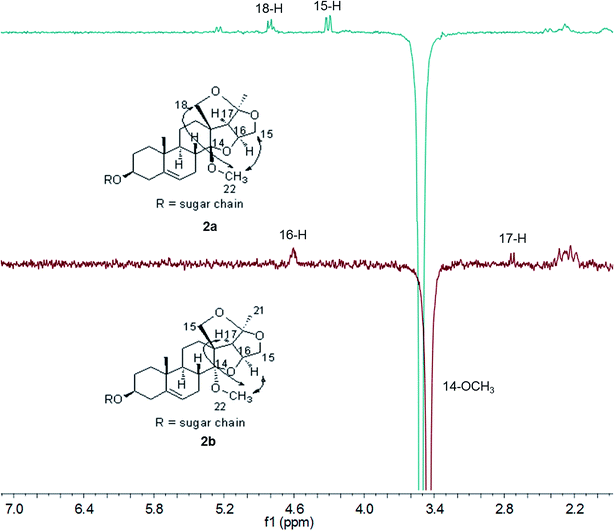
During the purification of compounds 1–6 by preparative HPLC, we found that they were unstable. Two discrete peaks always appeared in the HPLC by repeated preparative HPLC purification. Previous papers reported that hirundigenin was easy to transfer into anhydrohirundigenin by thermal dehydration or under acidic condition due to the presence of a free hemiketal hydroxyl group in its structure.15,16 Firstly, UPLC-MS/MS was applied to analyze their molecular formulas of the two discrete peaks. The result showed that they had the same molecular formulas, which excluded the presence of dehydration product (data not shown). Then, methylation of compounds 2 and 6 were performed under the condition of CH3I and NaH in anhydrous DMF. Four permethylated epimers, 14β-(2a and 6a) and 14α-OCH3 derivatives (2b and 6b), were obtained by semipreparative HPLC. Their chemical structures were identified by HR ESI-TOF MS, 1D and 2D-NMR experiments (Fig. S36–S67†). The relative configurations were determined by sel-NOE experiments (Fig. 5). These results revealed that hirundigosides E–J (1–6) exist in nature as epimers due to isomerization of the 14-hemiketal hydroxyl group, which also appeared in some other types of natural products containing this functionality.17,18
It is striking that only one set of resonance signals appeared in the 1H and 13C NMR spectra of compounds 1–6 when measured in CDCl3, while two sets of resonance signals were observed when measured in pyridine-d5. This may ascribe to lower molecular potential energy (G) of 14β-OH epimers than that of 14α-OH epimers. Since it is favorable for the formation of intramolecular hydrogen bond in 14β-OH epimer due to the proximate distance of hydroxyl group and oxygen atoms (1.86 nm) in 14β-OH epimer in CHCl3 and the difference of potential energy (ΔG) of 14α-OH epimer to 14β-OH epimer is about 0.73 kcal mol−1 for compound 1 (Fig. 6). Meanwhile, it is unfavorable for the formation of intramolecular hydrogen bond in pyridine and the ΔG of 14α-OH epimer to 14β-OH epimer is about −0.10 kcal mol−1. Also, CDCl3 is neither protic solvent nor Lewis acid and it can't induce the isomerization of hemiketal hydroxyl group in hirundigosides, while pyridine is Lewis base and it promotes the isomerization of hemiketal hydroxyl group.
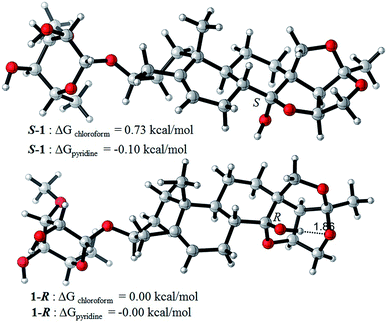 | ||
| Fig. 6 The difference of potential energy (ΔG) of 14α-OH (S-1) and 14β-OH epimer (1-R) in chloroform and pyridine. All calculations were performed using Gaussian09 suite of program. | ||
In addition, secondary anhydrohirundigenin-type glycosides (2d–2f and 6c–6f) and anhydrohirundigenin (2c) were obtained by mild acidic hydrolysis of compounds 2 and 6 followed by semipreparative HPLC purification in order to discuss the structure–activity relationship (SAR) of their anti-inflammatory activity. Among them, compounds 2d–2f and 6d–6f were new compounds and their chemical structures were determined by HR ESI-TOF MS, 1D- and 2D-NMR experiments (Fig. S36–S67, Tables S1–S3†). The known structures of 2c and 6c were identified through comparison of their physical and spectroscopic data with those reported previously.15,7
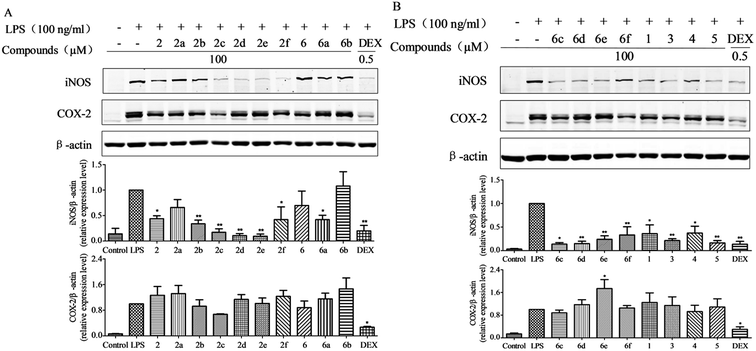
We evaluated the anti-inflammatory activity of hirundigosides E–J (1–6) and their derivatives (2a–2f and 6a–6f) by using a LPS-stimulated macrophage RAW264.7 cell model. As shown in Fig. 7(A and B), compounds 1–5, 2b–2f, 6a, and 6c–6f obviously inhibited LPS-induced iNOS protein expression compared to single LPS stimulation (blank control) in RAW264.7 cells. Meanwhile, results showed that all tested compounds couldn't remarkably suppress COX-2 protein expression. In contrast, compound 6e obviously increased COX-2 protein expression compared to single LPS stimulation. Structure–activity relationship (SAR) analysis revealed that anhydrohirundigenin-type C21 glycosides exhibited stronger inhibitory effects on the LPS-induced iNOS protein expression compared to that of hirundigenin-type C21 glycosides.
Mature macrophages are generally located tissues throughout the body, including spleen, lung, interstitial connective tissue, and liver, where they ingest and process foreign materials, dead cells and debris, and play a critical role in the initiation, maintenance and resolution of local inflammatory response.19 Upon soluble stimuli stimulation such as LPS, TNF-α and IL-1β, macrophages produce pro-inflammatory mediators, including nitric oxide (NO). NO is synthesized from the amino acid L-arginine using NADPH and molecular oxygen, and is one of the most versatile players in the immune system.20 However, overexpression of NO which is catalyzed by the enzymatic activity of nitric oxide synthases (NOS), is associated with a variety of pathological states. Therefore, NOS inhibitors could be as therapeutic agents for inflammatory diseases.21 Our studies revealed that most of the compounds could obviously inhibit LPS-induced iNOS protein expression in LPS-stimulated RAW264.7 cells. Results implied that these compounds probably possessed the anti-inflammatory properties and could, to a certain extent, suppress the NO release in the acute and chronic inflammatory response and had the potential to medicate anti-inflammatory action.
Experimental section
General experimental procedures
Optical rotations were obtained on a P-1020 digital polarimeter (Jasco Corporation). IR spectra were recorded on a JASCO FTIR-480 plus spectrometer. NMR spectra were measured on Bruker AV 300 and 600. The chemical shifts were given in ppm relative to chemical shifts of solvent resonances (C5D5N: δH 8.74/δC 150.3, CDCl3: δH 7.24/δC 77.2). HR-ESI-MS spectra were obtained on a Micromass Q-TOF mass spectrometer. A single-crystal X-ray diffraction was measured on Agilent Super-Nova. Analytical HPLC was performed on a Shimadzu LC-20AT Liquid Chromatograph with SPD-M20A Detector and an Alltech ELSD 2000 detector using a Phenomex C18 column (4.6 × 250 mm, 5 μm). Preparative HPLC was performed on a Shimadzu LC-6AD Liquid Chromatograph with SPD-20A Detector using an ODS column [YMC-Pack ODS-A (10.0 × 250 mm, 5 μm, 250 and 210 nm)]. Open column chromatography (CC) was performed using silica gel (200–300 mesh, Qingdao Haiyang Chemical Group Corp., Qingdao), ODS (50 μm, YMC, Japan), and Sephadex LH-20 (Pharmacia). TLC analysis was performed on pre-coated silica gel GF254 plates (Qingdao Haiyang Chemical Group Corp., Qingdao). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and other tissue culture reagents were purchased from Gibco BRL Co. (Grand Island, NY, USA). Lipopolysaccharide (LPS, Escherichia coli 055: B5) and dexamethason (DEX) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). iNOS and COX-2 antibodies were purchased from Cell Signaling Technology (Boston, MA, USA), and β-actin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). IRDye 800CW goat anti-mouse IgG (H + L) and IRDye 800CW goat anti-rabbit IgG (H + L) secondary antibodies were purchased from Li-COR Biotechnology (Lincoln, NE, USA).Plant material
The Chinese Medicine “Bai-Qian”, the dried roots of Cynanchum stauntonii, was obtained from Guangzhou Xiangxue pharmaceutical Co., Ltd. in November, 2012. It was identified by associated Prof. Zhang Ying, College of Pharmacy, Jinan University. A voucher specimen (CS201211-1) was deposited in the Institute of Traditional Chinese Medicine & Natural Products, Jinan University.Extraction and isolation
The air-dried and chopped roots of C. stauntonii (9.5 kg) were refluxed with 95% (v/v) aqueous EtOH (90 L, ×3). The extract was evaporated under reduced pressure to yield a dark-brown residue (ca. 1100 g). The residue was directly chromatographed over an open macroporous resin Diaion HP-20 column (ϕ 12.0 × 63.0 cm), eluted with gradient EtOH–H2O (0%, 30%, 50%, and 95%) to yield 4 fractions (A–D). The fraction D (93.0 g, 95%) was applied to silica gel column chromatography (ϕ 9.5 × 68.0 cm) using a gradient of mixtures of petroleum ether–EtOAc (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 98
0, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 95
2, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 9
5, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7
1, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 0
3, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) and EtOAc–CH3OH (95
100) and EtOAc–CH3OH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 9
5, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7
1, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 0
3, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) to yield 13 fractions (D1–D13) according to their TLC profiles and HPLC behavior. Fraction D10 (20.8 g) was subjected to open ODS CC (ϕ 4.0 × 30.0 cm) using a CH3OH–H2O gradient to afford 13 subfractions (D10-1–D10-13). Subfraction D10-4 (1.8 g) was separated by ODS CC using gradient solvents of CH3OH–H2O to yield 9 subfractions (D10-4-1–D10-4-9). Subfraction D10-4-5 was further purified by semipreparative RP-C18 HPLC with MeOH–H2O (50
100) to yield 13 fractions (D1–D13) according to their TLC profiles and HPLC behavior. Fraction D10 (20.8 g) was subjected to open ODS CC (ϕ 4.0 × 30.0 cm) using a CH3OH–H2O gradient to afford 13 subfractions (D10-1–D10-13). Subfraction D10-4 (1.8 g) was separated by ODS CC using gradient solvents of CH3OH–H2O to yield 9 subfractions (D10-4-1–D10-4-9). Subfraction D10-4-5 was further purified by semipreparative RP-C18 HPLC with MeOH–H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) to afford compound 1 (10.0 mg). Compound 4 (50.0 mg) was obtained from D10-4-9 by semipreparative RP-C18 HPLC using MeOH–H2O (60
50) to afford compound 1 (10.0 mg). Compound 4 (50.0 mg) was obtained from D10-4-9 by semipreparative RP-C18 HPLC using MeOH–H2O (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40). Subfraction D10-5 (2.4 g) was subjected to ODS CC using a stepwise gradient of MeOH in H2O from 60% to 80% to give 4 subfractions (D10-5-1–D10-5-4). Subfractions D10-5-1 was chromatographed over Sephadex LH-20 CC eluting with MeOH–H2O (70
40). Subfraction D10-5 (2.4 g) was subjected to ODS CC using a stepwise gradient of MeOH in H2O from 60% to 80% to give 4 subfractions (D10-5-1–D10-5-4). Subfractions D10-5-1 was chromatographed over Sephadex LH-20 CC eluting with MeOH–H2O (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to afford 5 fractions (D10-5-1-1–D10-5-1-5). Fraction D10-5-1-3 (77 mg) was purified by semipreparative RP-C18 HPLC with MeOH–H2O (70
30) to afford 5 fractions (D10-5-1-1–D10-5-1-5). Fraction D10-5-1-3 (77 mg) was purified by semipreparative RP-C18 HPLC with MeOH–H2O (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to afford compound 3 (38.0 mg). Compound 5 (48.0 mg) was obtained from D10-5-1-4 (237 mg) by semipreparative RP-C18 HPLC using MeCN–H2O (40
30) to afford compound 3 (38.0 mg). Compound 5 (48.0 mg) was obtained from D10-5-1-4 (237 mg) by semipreparative RP-C18 HPLC using MeCN–H2O (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60). Fraction D12 (24.0 g) was subjected to open ODS CC (ϕ 4.0 × 30.0 cm) using a CH3OH–H2O gradient to give 9 subfractions (D12-1–D12-9). Subfraction D12-2 (16.0 g) was chromatographed over Sephadex LH-20 CC eluting with MeOH–H2O (70
60). Fraction D12 (24.0 g) was subjected to open ODS CC (ϕ 4.0 × 30.0 cm) using a CH3OH–H2O gradient to give 9 subfractions (D12-1–D12-9). Subfraction D12-2 (16.0 g) was chromatographed over Sephadex LH-20 CC eluting with MeOH–H2O (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to afford 9 subfractions (D12-2-1–D12-2-9). Compound 6 (500 mg) was obtained from D12-2-2 (1.57 g) by preparative RP-C18 HPLC using MeOH–H2O (65
30) to afford 9 subfractions (D12-2-1–D12-2-9). Compound 6 (500 mg) was obtained from D12-2-2 (1.57 g) by preparative RP-C18 HPLC using MeOH–H2O (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35). Subfraction D12-3 (6.0 g) was chromatographed over silica gel CC (ϕ 2.5 × 25.0 cm) eluting with gradient CHCl3–MeOH (95
35). Subfraction D12-3 (6.0 g) was chromatographed over silica gel CC (ϕ 2.5 × 25.0 cm) eluting with gradient CHCl3–MeOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 9
5, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 85
1, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 8
15, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0
2, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) to yield 7 subfractions (D12-3-1–D12-3-7). Subfraction D12-3-2 (1.57 g) was subjected to Sephadex LH-20 CC and then purified by preparative RP-C18 HPLC with MeOH–H2O (70
100) to yield 7 subfractions (D12-3-1–D12-3-7). Subfraction D12-3-2 (1.57 g) was subjected to Sephadex LH-20 CC and then purified by preparative RP-C18 HPLC with MeOH–H2O (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to yield compound 2 (480 mg).
30) to yield compound 2 (480 mg).
Permethylation of hirundigoside F (2) and J (6)
To a solution of compound 2 (50 mg) in anhydrous DMF (3 mL) was added NaH. The reaction mixture was stirred for half an hour in ice bath and was added CH3I (1 mL). After 1 h, 5 mL of H2O was added to the reaction mixture to stop the reaction. The reaction mixture was extracted with CHCl3 (5 mL × 3). The organic layer was concentrated under reduced pressure to provide crude product, which was purified by semipreparative RP-HPLC using MeOH–H2O (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to afford compounds 2a and 2b. Permethyl derivatives of hirundigoside J (6a and 6b) were prepared in the same way as described above.
20) to afford compounds 2a and 2b. Permethyl derivatives of hirundigoside J (6a and 6b) were prepared in the same way as described above.
Mild acid hydrolysis of hirundigoside F (2) and J (6) and preparation of their secondary glycosides
To a solution of compound 2 (80 mg) in MeOH (2 mL) was added 0.05 N HCl (3 mL). The reaction mixture was stirred for 15 min at 70 °C and evaporated to remove MeOH under reduced pressure. The aqueous solution was extracted with CHCl3 (3 mL × 3). The recovered organic layers were concentrated to provide crude product, which was purified by semipreparative RP-HPLC using MeOH–H2O (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to afford anhydrohirundigenin (2c) and secondary anhydrohirundigenin-type glycosides (2d–2f). The secondary anhydrohirundigenin-type glycosides (6c–6f) derived from hirundigoside J (6) were prepared in the same way as described above.
30) to afford anhydrohirundigenin (2c) and secondary anhydrohirundigenin-type glycosides (2d–2f). The secondary anhydrohirundigenin-type glycosides (6c–6f) derived from hirundigoside J (6) were prepared in the same way as described above.
Hirundigoside E (1)
Colorless needle crystals (CHCl3/MeOH), [α]27D −15.2 (c = 0.5, CH3OH); IR (KBr) νmax 3441, 2939, 1706, 1455, 1379, and 1069 cm−1; ESI-MS (positive mode) m/z 545.9 [M + Na]+, HR ESI-MS (positive mode) m/z 545.2732 [M + Na]+ (calcd for C28H42O9Na, 545.2727); 1H (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Tables 1–3.Hirundigoside F (2)
Colorless amorphous powder; [α]27D −20.0 (c = 0.5, CH3OH); IR (KBr) νmax 3436, 2934, 1708, 1454, 1378, and 1063 cm−1; ESI-MS (positive mode) m/z 789.7 [M + Na]+, HR ESI-MS (positive mode) m/z 789.4027 [M + Na]+ (calcd for C40H62O14Na, 789.4037); 1H (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Tables 1–3.Hirundigoside G (3)
Colorless amorphous powder; [α]27D −35.6 (c = 0.5, CH3OH); IR (KBr) νmax 3445, 2976, 1692, 1514, 1389, and 1061 cm−1; ESI-MS (positive mode) m/z 803.9 [M + Na]+, HR ESI-MS (positive mode) m/z 803.4194 [M + Na]+ (calcd for C41H64O14Na, 803.4194); 1H (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Tables 1–3.Hirundigoside H (4)
Colorless amorphous powder; [α]27D −6.4 (c = 0.5, CH3OH); IR (KBr) νmax 3445, 2934, 1644, 1456, 1377, and 1073 cm−1; ESI-MS (positive mode) m/z 819.7 [M + Na]+, HR ESI-MS (positive mode) m/z 819.4133 [M + Na]+ (calcd for C41H64O15Na, 819.4143); 1H (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Tables 1–3.Hirundigoside I (5)
Colorless amorphous powder; [α]27D −22.4 (c = 0.5, CH3OH); IR (KBr) νmax 3445, 2936, 1455, 1375, and 1060 cm−1; ESI-MS (positive mode) m/z 977.8 [M + Na]+, HR ESI-MS (positive mode) m/z 977.5084 [M + Na]+ (calcd for C49H78O18Na, 778.5164); 1H (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Tables 1–3.Hirundigoside J (6)
Colorless amorphous powder; [α]27D −34.4 (c = 0.5, CH3OH); IR (KBr) νmax 3474, 2934, 1644, and 1080 cm−1; ESI-MS (positive mode) m/z 963.9 [M + Na]+, HR ESI-MS (positive mode) m/z 963.4912 [M + Na]+ (calcd for C48H76O18Na, 963.4929); 1H (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Tables 1–3.14β-OCH3-permethyl hirundigoside F (2a)
Colorless amorphous powder; [α]27D −67.9 (c = 0.5, CH3OH); IR (KBr) νmax 2934, 1455, 1380, and 1002 cm−1; ESI-MS (positive mode) m/z 845.9 [M + Na]+, HR ESI-MS (positive mode) m/z 845.4666 [M + Na]+ (calcd for C44H70O14Na, 845.4663); 1H (600 MHz, C5D5N) and 13C NMR (150 MHz, C5D5N) data, see Tables S1–S3.†14α-OCH3-permethyl hirundigoside F (2b)
Colorless amorphous powder; [α]27D −50.0 (c = 0.5, CH3OH); IR (KBr) νmax 2934, 1455, 1380, and 1002 cm−1; ESI-MS (positive mode) m/z 845.9 [M + Na]+, HR ESI-MS (positive mode) m/z 845.4647 [M + Na]+ (calcd for C44H70O14Na, 845.4663); 1H (600 MHz, C5D5N) and 13C NMR (150 MHz, C5D5N) data, see Tables S1–S3.†Anhydrohirundigenin β-D-canaropyranoside (2d)
Colorless amorphous powder; [α]27D +2.6 (c = 0.5, CH3OH); IR (KBr) νmax 3441, 2933, 1645, 1375, and 1068 cm−1; ESI-MS (positive mode) m/z 497.7 [M + Na]+, HR ESI-MS (positive mode) m/z 475.2697 [M + H]+ (calcd for C27H39O7, 475.2696); 1H (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Tables S1–S3.†Anhydrohirundigenin β-D-digitoxopyranosyl-(1 → 4)-β-D-canaropyranoside (2e)
Colorless amorphous powder; [α]27D −43.2 (c = 0.5, CH3OH); IR (KBr) νmax 3441, 2934, 1645, 1375, and 1068 cm−1; ESI-MS (positive mode) m/z 627.8 [M + Na]+, HR ESI-MS (positive mode) m/z 605.3336 [M + H]+ (calcd for C33H49O10, 605.3326); 1H (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Tables S1–S3.†Anhydrohirundigenin 3-O-α-L-cymaropyranosyl-(1 → 4)-β-D-digitoxopyranosyl-(1 → 4)-β-D-canaropyranoside (2f)
Colorless amorphous powder; [α]27D −71.4 (c = 0.5, CH3OH); IR (KBr) νmax 3441, 2934, 1645, 1375, and 1068 cm−1; ESI-MS (positive mode) m/z 771.9 [M + Na]+, HR ESI-MS (positive mode) m/z 771.3906 [M + Na]+ (calcd for C40H60O13Na, 771.3932); 1H (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Tables S1–S3.†14β-OCH3-permethyl hirundigoside J (6a)
Colorless amorphous powder; [α]27D −41.0 (c = 0.5, CH3OH); IR (KBr) νmax 2933, 1455, 1379, and 1061 cm−1; ESI-MS (positive mode) m/z 1020.1 [M + Na]+; 1H (600 MHz, C5D5N) and 13C NMR (150 MHz, C5D5N) data, see Tables S1–S3.†14α-OCH3-permethyl hirundigoside J (6b)
Colorless amorphous powder; [α]27D −37.7 (c = 0.5, CH3OH); IR (KBr) νmax 2934, 1455, 1380, and 1002 cm−1; ESI-MS (positive mode) m/z 1020.2 [M + Na]+; 1H (600 MHz, C5D5N) and 13C NMR (150 MHz, C5D5N) data, see Tables S1–S3.†Anhydrohirundigenin β-D-digitoxopyranosyl-(1 → 4)-β-D-thevetopyranoside (6d)
Colorless amorphous powder; [α]27D −8.3 (c = 0.5, CH3OH); IR (KBr) νmax 3441, 2934, 1645, 1375, and 1068 cm−1; ESI-MS (positive mode) m/z 657.9 [M + Na]+, HR ESI-MS (positive mode) m/z 657.3245 [M + Na]+ (calcd for C34H50O11Na, 657.3251); 1H (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Tables S1–S3.†Anhydrohirundigenin β-D-cymaropyranosyl-(1 → 4)-β-D-digitoxopyranosyl-(1 → 4)-β-D-thevetopyranoside (6e)
Colorless amorphous powder; [α]27D +4.7 (c = 0.5, CH3OH); IR (KBr) νmax 3441, 2934, 1645, 1375, and 1068 cm−1; ESI-MS (positive mode) m/z 802.1 [M + Na]+, HR ESI-MS (positive mode) m/z 801.4035 [M + Na]+ (calcd for C41H62O14Na, 801.4037); 1H (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Tables S1–S3.†Anhydrohirundigenin 3-O-α-L-diginopyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-β-D-digitoxopyranosyl-(1 → 4)-β-D-thevetopyranoside (6f)
Colorless amorphous powder; [α]27D − 33.5 (c = 0.5, CH3OH); IR (KBr) νmax 3441, 2933, 1645, 1375, and 1068 cm−1; ESI-MS (positive mode) m/z 946.0 [M + Na]+, HR ESI-MS (positive mode) m/z 945.4819 [M + Na]+ (calcd for C41H62O14Na, 945.4824); 1H (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Tables S1–S3.†X-ray crystallographic analysis of 1
Crystals of 1 were obtained in the mixture solvent of CHCl3 and CH3OH. Colorless blocks, C29H45O10, M = 553.65, monoclinic, space group P1211, a = 8.6095(14) Å, b = 7.8910(8) Å, c = 21.093(3) Å, V = 1412.5(3) Å, Z = 2, T = 293(2) K, μ(Cu Kα) = 0.804 mm−1, Dcalcd = 1.302 mg m−3, 3444 reflections collected (4.25 ≤ θ ≤ 62.66), 2669 unique (Rint = 0.0284), which were used in all calculations. The final R1 was 0.0552[I > 2σ(I)] and wR2 was 0.1432[I > 2 sigma(I)]. Data collection was performed in a SMART CCD by using graphite monochromated radiation (l = 1.54184). The structure of compound 1 was solved by the direct methods (SHELXTL version 6.1) and refined by full-matrix least-squares on F2. Crystallographic data (excluding structure factors) for compound 1 (Cu Kα radiation) reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 1433862.Computational calculations
All calculations were performed using Gaussian09 suite of program.22 Density functional theory method was used, employing uB3LYP hybrid functional.23,24 Geometry optimization was done using a combined basis set 6-31G(d). Frequency calculation was done at the same level of theory as geometry optimization, to confirm the stationary points to be minima. Single point energy calculations were done using B3LYP method at a larger basis set 6-31++G(d,p). Solvent effect was accounted for using self-consistent reaction field (SCRF) method using SMD model and UAKS radii.25,26 Chloroform and pyridine were used as solvents.Cells and treatments
The murine macrophage cell line RAW264.7 (American Type Culture Collection, Manassas, VA, USA) cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) FBS (Gibco BRL Co, Grand Island, NY, USA), penicillin G (100 units per ml), streptomycin (100 mg mL−1), and L-glutamine (2 mM) (Gibco BRL Co, Grand Island, NY, USA) in a 5% CO2-humidified incubator at 37 °C. All of tested compounds were dissolved in DMSO in 100 mM and work concentration was 100 μM (DMSO concentration was less than 0.1% in assay). To analysis the anti-inflammatory activity of these compounds derived from the dried roots of Cynanchum stauntonii. RAW264.7 cells were plated at a density of 8 × 104 cells per well in 24-well plates for 24 h. Cells were pretreated for 1 h with the test compounds or DEX before treatment with 100 ng mL−1 LPS. DEX was chosen as positive control in this study. Cells stimulated by LPS without any intervention were observed as blank control. Cells incubated by DMEM medium were as normal control.Western blot analysis
Total proteins were prepared from RAW264.7 cells through the use of RIPA lysis buffer (Cell Signaling technology, Boston, MA, USA) and protease inhibitors (Roche Applied Science, Germany). Lysate protein (30 μg per lane) was separated by 10% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (GE Healthcare Life Sciences, Buckinghamshire, UK). The membrane was blocked for 1 h with 5% skimmed milk, and then incubated with the primary antibody (iNOS and COX-2) and mouse antibodies specific for β-actin at 4 °C for overnight. The antigen–antibody complexes were visualized using IRDye 800CW goat anti-mouse IgG (H + L) or IRDye 800CW goat anti-rabbit IgG (H + L) secondary antibodies (Li-COR, Lincoln, NE, USA). The protein expression level was quantitated using Odyssey v3.0 software (Li-COR, USA).Statistical evaluation
All data are given as the mean ± standard deviation for the number of experiments. Statistical significance was compared each treated with compounds group with the blank control and determined by one-way analysis of variance (ANOVA) using SPSS statistical program. P values less than 0.05 was accepted as statistically significant.Acknowledgements
We are grateful to the State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology for the anti-inflammation activity testing. We are also grateful to Mr T. Shi, Mr J. Geng, and Dr Y. Yu for the HR ESI-MS and NMR measurements. Part of the chemical work was accomplished in Guangzhou Xiangxue Pharmaceutical Ltd. Co.References
- The State Pharmacopoeia Commission of PRC, Pharmacopoeia of the People's Republic of China, China Medical Science and Technology Press, Beijing, China, 2010, vol. 1, p. 101 Search PubMed
.
- M. Zhang, J. S. Wang, J. Luo, D. D. Wei and L. Y. Kong, Nat. Prod. Res., 2013, 27, 176–180 CrossRef CAS PubMed
.
- M. Shibano, A. Misaka, K. Sugiyama, M. Taniguchi and K. Baba, Phytochem. Lett., 2012, 5, 304–308 CrossRef CAS
.
- Y. M. Li, L. H. Wang, S. L. Li, X. Y. Chen, Y. M. Shen, Z. K. Zhang, H. P. He, W. B. Xu, Y. L. Shu, G. D. Liang, R. X. Fang and X. J. Hao, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8083–8088 CrossRef CAS PubMed
.
- C. Z. Lai, J. X. Liu, S. W. Pang, Y. Dai, H. Zhou, Z. Q. Mu, J. Wu, J. S. Tang, L. Liu and X. S. Yao, Phytochem. Lett., 2016, 16, 38–46 CrossRef CAS
.
- M. Lavault, P. Richomme and J. Bruneton, Fitoterapia, 1999, 70, 216–220 CrossRef CAS
.
- P. Wang, H. L. Qin, L. Zhang, Z. H. Li, Y. H. Wang and H. B. Zhu, Planta Med., 2004, 70, 1075–1079 CrossRef CAS PubMed
.
- J. L. Li, J. Zhou, Z. H. Chen, S. Y. Guo, C. Q. Li and W. M. Zhao, J. Nat. Prod., 2015, 78, 1548–1555 CrossRef CAS PubMed
.
- M. H. Fu, Z. J. Wang, H. J. Yang, M. Maimai, J. Fang, L. Y. Tang and L. Yang, Chin. Chem. Lett., 2007, 18, 415–417 CrossRef CAS
.
- N. Toshiyuki, F. Tsutomu, O. Naoko, H. Wataru, N. Junko and O. Tadatake, J. Appl. Glycosci., 1997, 44, 175–181 Search PubMed
.
- B. Malte and H.-U. Reißig, Eur. J. Org. Chem., 2009, 3595–3604 Search PubMed
.
- H. Hidekazu, A. Hideaki, K. Keisuke and A. Hiroyuki, Chem. Pharm. Bull., 2010, 58, 1411–1418 CrossRef
.
- J. Q. Yu, A. J. Deng and H. L. Qin, Steroids, 2013, 78, 79–90 CrossRef CAS PubMed
.
- N. Q. Zhu, M. F. Wang, H. Kikuzaki, N. Nakatani and C. T. Ho, Phytochemistry, 1999, 52, 1351–1355 CrossRef CAS PubMed
.
- O. Kennard, J. K. Fawcett, D. G. Watson and K. Ann, Tetrahedron Lett., 1968, 3799–3804 CrossRef CAS
.
- K. Stockel, W. Stocklin and T. Reichstein, Helv. Chim. Acta, 1969, 52, 147 Search PubMed
.
- P. P. Yadav, A. Arora, H. K. Bid, R. R. Konwar and S. Kanojiya, Tetrahedron Lett., 2007, 48, 7194–7198 CrossRef CAS
.
- S. Y. Cheng, Z. H. Wen, S. K. Wang, S. F. Chiou, C. H. Hsu, C. F. Dai, M. Y. Chiang and C. Y. Duh, J. Nat. Prod., 2009, 72, 152–155 CrossRef CAS
.
- P. J. Murray and T. A. Wynn, Nat. Rev. Immunol., 2011, 11, 723–737 CrossRef CAS PubMed
.
- C. Bogdan, Nat. Immunol., 2001, 2, 907–916 CrossRef CAS PubMed
.
- A. C. Tinker and A. V. Wallace, Curr. Top. Med. Chem., 2006, 6, 77–92 CrossRef CAS PubMed
.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford, CT, USA, 2009 Search PubMed
.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS
.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS
.
- V. Barone and M. Cossi, J. Phys. Chem. A, 1998, 102, 1995–2001 CrossRef CAS
.
- M. Cossi, N. Rega, G. Scalmani and V. Barone, J. Comput. Chem., 2003, 24, 669–681 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: The 1D and 2D NMR spectra of compounds 1–6, 2d–2f and 6d–6f (Fig. S1–S67). Assignments of the 1H and 13C NMR data for compounds 2d–2f and 6d–6f (Tables S1–S3). CCDC 1433862. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra11957c |
| ‡ Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |