Delavatine A, a structurally unusual cyclopenta[de]isoquinoline alkaloid from Incarvillea delavayi†
Zhongyin Zhangab,
Fan Yangb,
Jian-Jun Fub,
Yun-Heng Shen*a,
Weiwei He*b and
Wei-Dong Zhang*ab
aDepartment of Phytochemistry, School of Pharmacy, Second Military Medical University, Shanghai 200433, P. R. China. E-mail: shenyunheng@hotmail.com; wdzhangy@hotmail.com
bSchool of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, China
First published on 6th July 2016
Abstract
Natural products have long been an important source for the discovery of biologically active compounds, in particular, drug leads. Delavatine A (1), a structurally unusual cyclopenta[de]isoquinoline alkaloid, was isolated from Incarvillea delavayi. Extensive spectroscopic analysis, in combination with biogenetic speculation, established the structure of 1. Delavatine A (1) exhibited considerable cytotoxicity against a number of cancer cell lines with IC50 values in the range of 4.28 to 6.61 μg mL−1.
Introduction
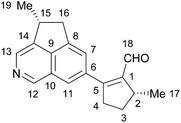
Natural products have long been an unparalleled source of drugs. Incarvillea species (Bignoniaceae) are flowering plants growing at high altitudes, which have been used in traditional Chinese herbal medicine for centuries. Recently, actinidine-type monoterpene alkaloids with potent antinociceptive and anti-inflammatory activities were isolated from Incarvillea species,1–4 inspiring our considerable interest in the chemical constituents of Incarvillea plants. Incarvillea delavayi Bureau et Franchet is a mountain flowering plant native to Yunnan and Sichuan provinces in Southwest China.5 In Chinese herbal medicine, the roots of I. delavayi are commonly used to treat dizziness and anemia and stimulate lactation.6 We isolated a benzofuranone dimer and iridoids from this plant previously.7,8 Based on our continuous investigations of the chemical constituents of Incarvillea species, we disclose here the isolation of delavatine A (1, Fig. 1) from I. delavayi, a structurally unusual alkaloid featuring an unique cyclopenta[de]isoquinoline skeleton with a methylcyclopentenaldehyde substituent at the isoquinoline C6 position. The structural connectivity of 1 was determined by spectroscopic methods, and its possible stereochemical configuration was proposed based on a biogenetic hypothesis. Delavatine A (1) displayed considerable cytotoxicity against a number of human cancer cell lines (MCF7, HCT116, SKOV3, SMMC-7721, and HeLa cells) with IC50 values in the range of 4.28 to 6.61 μg mL−1.Results and discussion
The chloroform (CHCl3) fraction of the 80% ethanol extract of the whole plant of I. delavayi was repeatedly purified by using silica gel chromatography, reverse-phase silica gel chromatography, and Sephadex LH-20 column chromatography, to afford delavatine A (1) in 0.0071% overall yield.Delavatine A (1) was obtained as pale yellow oil with an optical rotation {[α]20D = +56 (c = 0.05 in CHCl3)}, and gave positive reaction to Dragendorff's reagent. The UV spectrum of 1 showed absorption maxima at 249.2 and 273.4 nm. Its molecular formula was established to be C19H19NO by analysis of HR-ESI-MS (m/z [M + H]+ 278.1546, calcd for C19H20NO+ 278.1545), requiring a degree of unsaturation of 11. The IR spectrum of 1 showed the presence of the absorption bands for methyl (2958, 2869, 1456, 1375 cm−1), aldehyde carbonyl (1700 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond (1665 cm−1), and benzene ring (1617 cm−1).
C double bond (1665 cm−1), and benzene ring (1617 cm−1).
In the 1H NMR spectrum of 1 (data shown in Table 1), several characteristic proton signals, including an aldehyde proton at δH 9.82 (s), four aromatic protons at δH 7.43 (s), 7.71 (s), 9.11 (s), and 8.47 (s), two doublet methyls at δH 1.51 (d, J = 7.2 Hz) and 1.25 (d, J = 6.6 Hz), were observed. Additionally, the 1H NMR spectrum of 1 also displayed two groups of doublet of double doublets proton signals at δH 3.11 (ddd, J = 13.8, 8.4, 1.8 Hz) and 2.96 (ddd, J = 13.8, 9.6, 5.6 Hz), together with two groups of double doublet protons at δH 3.69 (dd, J = 17.4, 7.8 Hz) and 2.98 (dd, J = 17.4, 3.6 Hz). The remaining protons occurred at δH 3.31, 2.29, 1.68, and 3.90 as multiplet signals, resonating from either a methine or methylene group. The 13C and DEPT NMR spectra (data shown in Table 1) exhibited nineteen carbon resonances, which was sorted into two methyls, three methylenes, seven methines, and seven quaternary carbons. Detailed analysis of the 1H and 13C NMR data (Table 1) disclosed the presence of several characteristic functionalities, including one aldehyde group (δH 9.82, δC 190.3), one tetra-substituted double bond (δC 144.4, 162.3), and one tetra-substituted benzene ring (δH 7.43, 7.71, δC 136.3, 123.5, 144.4, 141.8, 125.9, and 122.4) fused with one pyridine ring (δH 9.11 and 8.47, δC 146.8, 137.3, and 143.7). All proton and carbon resonances were assigned by interpretation of the 1H and 13C NMR data, together with 2D NMR experiments.
| No. | δH (J in Hz) | δC | No. | δH (J in Hz) | δC |
|---|---|---|---|---|---|
| a In CDCl3, 600 MHz for 1H NMR, 150 MHz for 13C NMR. | |||||
| 1 | 144.4 | 10 | 125.9 | ||
| 2 | 3.31 m | 39.2 | 11 | 7.71 s | 122.4 |
| 3a | 2.29 m | 30.9 | 12 | 9.11 s | 146.8 |
| 3b | 1.68 m | 13 | 8.47 s | 137.3 | |
| 4a | 3.11 ddd (13.8, 8.4, 1.8) | 38.1 | 14 | 143.7 | |
| 4b | 2.96 ddd (13.8, 9.6, 5.6) | 15 | 3.90 m | 37.4 | |
| 5 | 162.3 | 16a | 3.69 dd (17.4, 7.8) | 39.6 | |
| 6 | 136.3 | 16b | 2.98 dd (17.4, 3.6) | ||
| 7 | 7.43 s | 123.5 | 17 | 1.51 d (7.2) | 19.5 |
| 8 | 144.4 | 18 | 9.82 s | 190.3 | |
| 9 | 141.8 | 19 | 1.25 d (6.6) | 21.5 | |
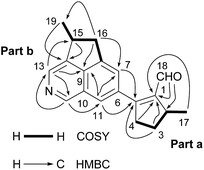
More detailed information of the structure of 1 was obtained due to analysis of 2D NMR spectra (Fig. 2). In the 1H–1H correlation spectroscopy (COSY) NMR spectrum of 1, the direct correlations of CH3-17/H-2/H2-3/H2-4 were observed (Fig. 2). Moreover, the heteronuclear multiple-bond correlation (HMBC) NMR spectrum of 1 exhibited key correlations from the aldehyde proton at δH 9.82 to C-1 (δC 144.4) and C-2 (δC 39.2), from H2-4 at δH 3.11 and 2.96 to C-1 and C-5 (δC 162.3), and from CH3-17 at δH 1.51 to C-1, established a α,β-unsaturated aldehyde fragment, and further constructed a methylcyclopentenaldehyde moiety (Fig. 2, part a). The Dragendorff's reagent positive reaction of 1, in combination with the presence of benzene ring and pyridine ring, led to the speculation that there is an isoquinoline core in the structure of 1. The HMBC correlations of H-7 at δH 7.43 to C-9 (δC 141.8) and C-11 (δC 122.4), of H-11 at δH 7.71 to C-7 (δC 123.5), C-9 (δC 141.8), and C-12 (δC 146.8), of H-12 at δH 9.11 to C-13 (δC 137.3) and C-11, and of H-13 at δH 8.47 to C-9 and C-12 (δC 146.8), confirmed the presence of the isoquinoline core. Interpretation of the 1H–1H COSY spectrum of 1 also supported the presence of the fragment CH3-19/CH-15/CH2-16, which was linked to the isoquinoline core by two C–C bonds (C-8/C-16 and C-14/C-15) due to key HMBC correlations of CH3-19 at δH 1.25 with C-14 (δC 143.7), of H2-16 at δH 3.69 and 2.98 with C-7, C-9, and C-19, and of H-13 with C-15 (δC 37.4), thus established a methylcyclopentaisoquinoline moiety (Fig. 2, part b). In the HMBC spectrum, the long-range correlations of H-7 and H-11 with C-5 strongly supported the linkage of the methylcyclopentenaldehyde moiety (part a) and methylcyclopentaisoquinoline moiety (part b) through C-5/C-6 bond, and established the planar structure of 1 as shown in Fig. 1.
The nuclear Overhauser effect spectroscopy (NOESY) NMR spectrum of 1 exhibited the correlations of H-7 with the aldehyde proton (H-18) and H-16 at δH 3.69, of H-11 with H-4 at δH 2.96 and H-12, and of CH3-19 with H-13 (Fig. 3), which also supported the planar structure of 1. Because the two stereogenic centers are remote from each other, it is difficult to determine the relative configuration of 1 by interpretation of the NOESY spectrum.
The configuration issue of delavatine A (1) is certainly relevant to its biogenesis. Notably, monoterpenoid alkaloids and iridoids are two major classes of chemical constituents of Incarvillea species, and both arise from iridodial or its diastereoisomers.9,10 Thus, we speculated that 8-epi-iridodial10 (2, Scheme 1) may be the biosynthetic precursor of 1. A plausible biogenetic pathway is depicted in Scheme 1. The key transformation in this pathway is an intermolecular Diels–Alder cycloaddition11,12 between two 8-epi-iridodial derivatives in high oxidation states. Oxidative aromatization followed by ammonia condensation assembles the structure of 1. The two tertiary carbons should arise from the same region of precursor 2 and thus have the same configurations, which are postulated to be 2R and 15R on the basis of the stereochemistry of 2. Obviously, iridodial, instead of 2, can be the possible biosynthetic precursor of 1 as well; in that case, 1 should possess an absolute configuration of 2S,15S. We previously reported a structurally related natural product hybrid incarviatone A13 isolated from the same batch of plant material as 1, which possessed two tertiary chiral centers at C-2 and C-13 similar to those of 1 at C-2 and C-15. Very recently, Lei et al. reported the total synthesis of incarviatone A14 and determined its absolute configuration to be 2R,13R. Because incarviatone A and delavatine A presumably share similar biosynthetic pathways, we propose that delavatine A should possess a configuration of 2R,15R, as shown in Fig. 1. A chemical synthesis is desired for unambiguous stereochemical assignment of this natural product.
We examined the effect of delavatine A (1) on cell viability of a number of cancer cell lines, such as MCF7 human breast cancer cells, HCT116 human colon cancer cells, SKOV3 human ovarian cancer cells, SMMC-7721 human liver cancer cells, and HeLa human cervical cancer cells, using celastrol (also named tripterine) as a positive control.15,16 Cell viability was measured by the Cell Counting Kit-8 (CCK-8) assay, and the 50% inhibitory concentrations (IC50) for 24 h treatment were shown in Table 2. 1 displayed considerable cellular toxicity, and the IC50 values varied in the range of 4.28 to 6.61 μg mL−1.
| Cancer cells | Delavatine A (1) IC50 (μg mL−1) | Celastrol IC50 (μg mL−1) |
|---|---|---|
| MCF7 | 6.19 ± 0.31 | 0.92 ± 0.50 |
| HCT116 | 5.52 ± 0.33 | 1.44 ± 0.64 |
| SKOV3 | 4.28 ± 0.29 | 1.77 ± 0.78 |
| SMMC-7721 | 4.79 ± 0.30 | 0.59 ± 0.49 |
| HeLa | 6.61 ± 0.34 | 0.78 ± 0.53 |
Experimental
General experimental procedures
Plant material
The whole plants of Incarvillea delavayi were collected in Eryuan county, Yunnan province, P. R. China, in July 2006, and authenticated by Prof. Li-Shan Xie of Kunming Institute of Botany, the Chinese Academy of Sciences. A voucher specimen (no. 2006071003) is deposited in School of Pharmacy, Second Military Medical University.Extraction and isolation
The dried whole plants of I. delavayi (17 kg) were refluxed with 80% EtOH for three times (each 1.5 h). The combined extract was concentrated to a small volume (about 1 L), and then dissolved in 2% HCl and filtered. The filtrate was extracted with EtOAc to de-fat, and subsequently adjusted to pH 9–10 by adding 10% NaOH, and then extracted with CHCl3 to give CHCl3 fraction. The CHCl3 fraction (350 g) was subjected to silica gel column chromatography (CC) with gradient CHCl3–acetone (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–0
0–0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) to afford 6 fractions (F1–F6). Fraction F1 (25 g) was divided into 12 subfractions (F1-1–F1-12) by reverse-phase CC (YMC-Gel ODS-A, 50 μm) eluting with gradient MeOH–H2O (from 50% to 90% MeOH) on a BUCHI RP-MPLC instrument. Subsequently, subfraction F1-8 (5 g) was purified by Sephadex LH-20 CC (CHCl3:
100) to afford 6 fractions (F1–F6). Fraction F1 (25 g) was divided into 12 subfractions (F1-1–F1-12) by reverse-phase CC (YMC-Gel ODS-A, 50 μm) eluting with gradient MeOH–H2O (from 50% to 90% MeOH) on a BUCHI RP-MPLC instrument. Subsequently, subfraction F1-8 (5 g) was purified by Sephadex LH-20 CC (CHCl3:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 1:
MeOH, 1:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield 5 subfractions (F1-8-1–F1-8-5). F1-8-4 (2.7 g) was chromatographed on a silica gel column eluting with CHCl3–MeOH (20:
1) to yield 5 subfractions (F1-8-1–F1-8-5). F1-8-4 (2.7 g) was chromatographed on a silica gel column eluting with CHCl3–MeOH (20:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford delavatine A (1.2 g).
1) to afford delavatine A (1.2 g).
Cell culture
Human cancer cell lines MCF7, HCT116, SKOV3, SMMC-7721, and HeLa were purchased from the cell bank of the Chinese Academy of Sciences, Shanghai, China. MCF7 and HCT116 cells were cultured in DMEM media (Gibco™, Thermos Fisher Scientific, 11995-065) with 10% Fetal Bovine Serum (FBS, Biological Industries, 04-001-1A) and supplemented with 1% penicillin/streptomycin (Gibco™, Thermos Fisher Scientific, 15070-063). SKOV3, SMMC-7721, and HeLa cells were cultured in RPMI 1640 media (Gibco™, Thermos Fisher Scientific, 11875-093) with 10% FBS and supplemented with 1% penicillin/streptomycin. All cells were maintained at 37 °C with 5% of CO2 in a humidified incubator.Measurement of cytotoxicity
The cells were plated in a 96-well culture plate at a density of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per well. The tested compounds of various concentrations (0–13.87 μg mL−1) in DMSO (Sigma-Aldrich, D4540-500 mL) were added into the wells. The control compound celastrol was tested with concentrations in the range of 0–4.51 μg mL−1. Cell cytotoxicity was determined after treatment with compounds for 24 h. Cell viability was measured by using the Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc., USA) according to the manufacturer's instructions, and absorbance at 450 nm was measured by Synergy 2 multi-mode plate reader (BioTek, USA). Data were collected by Gen5™ Reader Control and Data Analysis Software (BioTek, USA). At each concentration, the inhibition rates were independently measured on five different wells of cancer cells. All the data were processed by Prism 5.0 (GraphPad Software, USA) for calculating IC50 values.
000 per well. The tested compounds of various concentrations (0–13.87 μg mL−1) in DMSO (Sigma-Aldrich, D4540-500 mL) were added into the wells. The control compound celastrol was tested with concentrations in the range of 0–4.51 μg mL−1. Cell cytotoxicity was determined after treatment with compounds for 24 h. Cell viability was measured by using the Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc., USA) according to the manufacturer's instructions, and absorbance at 450 nm was measured by Synergy 2 multi-mode plate reader (BioTek, USA). Data were collected by Gen5™ Reader Control and Data Analysis Software (BioTek, USA). At each concentration, the inhibition rates were independently measured on five different wells of cancer cells. All the data were processed by Prism 5.0 (GraphPad Software, USA) for calculating IC50 values.
Conclusions
Isoquinoline alkaloid is a type of important natural product with diverse unique structures and bioactivities.17 We discovered a structurally unusual cyclopenta[de]isoquinoline alkaloid delavatine A (1) from Incarvillea delavayi, a plant long used in Chinese traditional medicine. The structural connectivity of the natural product was established by spectroscopic analysis. The two stereogenic centers were proposed to possess the configurations of 2R and 15R, based on the biogenetic speculation and the stereochemistry of a highly relevant natural product. Cyclopenta[de]isoquinoline core was only reported as synthetic intermediate in a literature.18 To our knowledge, this is the first report of methylcyclopentenaldehyde substituted cyclopenta[de]isoquinoline skeleton in a naturally occurring molecule. Delavatine A (1) displayed considerable cytotoxicity against a number of cancer cell lines and may potentially serve as a lead compound for anticancer drug discovery.Acknowledgements
The work was supported by Professor of Chang Jiang Scholars Program, Ministry of Science & Technology (2013CB836900), National Natural Science Foundation of China (81230090, 81520108030, 81573318, 81373301, 81302658, 81502956, and 21572064), Shanghai Engineering Research Center for the Preparation of Bioactive Natural Products (10DZ2251300), the Scientific Foundation of Shanghai China (12401900801 and 13401900101), Shanghai Science and Technology Commission (15PJ1401300), National Major Project of China (2011ZX09307-002-03), and the National Key Technology R&D Program of China (2012BAI29B06).Notes and references
- M. Nakamura, Y. M. Chi, W. M. Yan, Y. Nakasugi, T. Yoshizawa, N. Irino, F. Hashimoto, J. Kinjo, T. Nohara and S. Sakurada, J. Nat. Prod., 1999, 62, 1293–1294 CrossRef CAS.
- M. Nakamura, K. Kido, J. Kinjo and T. Nohara, Phytochemistry, 2000, 53, 253–256 CrossRef CAS PubMed.
- M. Nakumura, K. Kido, J. Kinjo and T. Nohara, Chem. Pharm. Bull., 2000, 48, 1826–1827 CrossRef.
- M. Nakamura, Y. M. Chi, W. M. Yan, A. Yonezawa, Y. Nakasugi, T. Yoshizawa, F. Hashimoto, J. Kinjo, T. Nohara and S. Sakurada, Planta Med., 2001, 67, 114–117 CrossRef CAS PubMed.
- W. C. Wang, in Flora of China: Bignoniaceae, Science press, Beijing, 1990, vol. 69, p. 46 Search PubMed.
- Editorial Committee, Chinese Herb, Shanghai Science and Technology Publisher, Shanghai, 1999, vol. 21, pp. 6435–6436 Search PubMed.
- Y. Q. Chen, Y. H. Shen, Y. Q. Su, L. Y. Kong and W. D. Zhang, Chem. Biodiversity, 2009, 6, 779–783 CAS.
- T. Lu, Y. H. Shen, M. Lu, J. Tang, L. Shan, R. H. Liu, H. L. Li and W. D. Zhang, Helv. Chim. Acta, 2009, 92, 768–773 CrossRef CAS.
- M. I. Sampaio-Santos and M. A. C. Kaplan, J. Braz. Chem. Soc., 2001, 12, 144–153 CrossRef CAS.
- S. Uesato, S. Ueda, K. Kobayashi, M. Miyauchi, H. Itoh and H. Inouye, Phytochemistry, 1986, 25, 2309–2314 CrossRef CAS.
- J. Deng, S. Zhou, W. Zhang, J. Li, R. Li and A. Li, J. Am. Chem. Soc., 2014, 136, 8185–8188 CrossRef CAS PubMed.
- C. Wan, J. Deng, H. Liu, M. Bian and A. Li, Sci. China: Chem., 2014, 57, 926–929 CrossRef CAS.
- Y. H. Shen, Y. Ding, T. Lu, X. C. Li, J. M. Tian, H. L. Li, L. Shan, D. Ferreira, I. A. Khan and W. D. Zhang, RSC Adv., 2012, 2, 4175–4180 RSC.
- B. Hong, C. Li, Z. Wang, J. Chen, H. Li and X. Lei, J. Am. Chem. Soc., 2015, 137, 11946–11949 CrossRef CAS PubMed.
- O. Ngassapa, D. D. Soejarto, J. M. Pezzuto and N. R. Farnsworth, J. Nat. Prod., 1994, 57, 1–8 CrossRef CAS.
- V. R. Yadav, S. Prasad, B. Sung, R. Kannappan and B. B. Aggarwal, Toxins, 2010, 2, 2428–2466 CrossRef CAS PubMed.
- K. W. Bentley, Nat. Prod. Rep., 2006, 23, 444–463 RSC.
- M. D. Clayton, Z. Marcinow and P. W. Rabidecau, Tetrahedron Lett., 1998, 39, 9127–9130 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11915h |
| This journal is © The Royal Society of Chemistry 2016 |