Folate-decorated redox/pH dual-responsive degradable prodrug micelles for tumor triggered targeted drug delivery†
Abstract
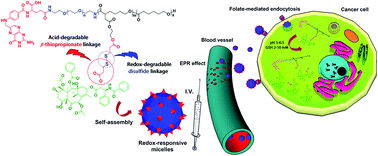
To address the obstacles facing the clinical use of paclitaxel, including poor water solubility, side effects and lack of tumor selectivity, a novel folate-decorated and redox and pH dual-responsive micellar drug delivery system was developed based on folate-poly(ethylene glycol)-b-poly((α-paclitaxel-SS-caprolactone)-co-caprolactone) (i.e., FA-PEG-b-P((PTX-SS-CL)-co-CL)) conjugates with thiol and acid-cleavable linkages. The paclitaxel (PTX) conjugated amphiphilic block copolymer prodrug was self-assembled in phosphate buffer (pH 7.4, 0.1 M) into nanosized spherical micelles (∼96.5 nm). In vitro release studies demonstrated that the prodrug micelles are relatively stable at normal physiologic conditions but susceptible to tumor-relevant reductive and acidic conditions which would trigger the release of chemically loaded drugs. Notably, folate-decorated PTX prodrug micelles based on FA-PEG-b-P((PTX-SS-CL)-co-CL) conjugates displayed apparent targetability to folate receptor-overexpressing HeLa cells. MTT assays showed that the therapeutic efficacy of these micelles against HeLa cancer cells (IC50 = 0.75 μg mL−1) was enhanced compared with free PTX (IC50 = 0.87 μg mL−1). These results suggest that FA-PEG-b-P((PTX-SS-CL)-co-CL) conjugates may offer a promising strategy for PTX delivery in the treatment of various tumors, with enhanced efficacy and fewer adverse effects.

 Please wait while we load your content...
Please wait while we load your content...