New short-channel SBA-15 mesoporous silicas functionalized with polyazamacrocyclic ligands for selective capturing of palladium ions in HNO3 media†
Fengcheng Wua,
Gang Ye*ab,
Yuekun Liuac,
Rong Yia,
Xiaomei Huoa,
Yuexiang Lua and
Jing Chen*ab
aInstitute of Nuclear and New Energy Technology (INET), Collaborative Innovation Center of Advanced Nuclear Energy Technology, Tsinghua University, Beijing 100084, China. E-mail: yegang@mail.tsinghua.edu.cn; jingxia@tsinghua.edu.cn
bBeijing Key Lab of Radioactive Waste Treatment, Tsinghua University, Beijing 100084, China
cDepartment of Engineering Physics, Tsinghua University, Beijing 100084, China
First published on 7th July 2016
Abstract
In this study, a new kind of short-channel SBA-15 mesoporous silica decorated with polyazamacrocyclic ligands was developed, showing selective binding ability to palladium ions based on host–guest interaction. The established synthesis protocol involved the co-condensation synthesis of an SBA-15 precursor with halogen atoms uniformly incorporated in the mesoporous silica matrix, followed by the anchoring of 1,4,7,10-teraazacyclododecane (Cyclen) ligands via post-grafting. Due to the short straight channels and large pore size facilitating the diffusion of the molecules and ions, the mesoporous silicas were found to possess a high density of the functional Cyclen ligands, as well as high adsorption capacity of Pd(II) in HNO3 solutions. The structure and morphology of the Cyclen functionalized mesoporous silicas were fully characterized. And, the adsorption behavior toward Pd(II) was investigated combined with the theoretical interpretation of the experimental data based on typical kinetic equations, isotherm models and thermodynamic equations. Furthermore, the detailed coordination mechanism between the Cyclen ligands and Pd(II) was examined by high resolution X-ray photoelectron spectroscopy (XPS). A suggested mechanism involving the synergistic effect of four cyclic amines in the Cyclen ligands was proposed to describe the coordination to Pd(II) in HNO3 solutions. Overall, this work provides a facile and effective pathway to build polyazamacrocycle ligand decorated mesoporous silicas with short-channels and large pores, which might be potentially used for molecule recognition and selective enrichment of precious metals.
Introduction
Since the innovative work of the Mobil scientists in the early 1990s,1,2 considerable research interests have been evoked on ordered mesoporous silicas (OMSs).3–5 Due to the large specific surface area, uniform channel structure, tunable pore size and favorable chemical–physical stability, these fascinating materials hold great promise for diverse applications such as catalysis, adsorption,6,7 gas storage,8 controlled-release systems,9,10 etc. In particular, by introducing organic ligands through approaches like post-grafting or co-condensation, the functionalized OMSs are regarded as ideal candidates for the adsorption of heavy metals,11–14 radionuclides,15–17 organic dyes18 and noble metals,19 with the purpose of environmental decontamination or resource reclamation.The control of morphology and internal channel structure is one of the main subjects in this rapidly developing research field.20,21 Regarding the adsorption application, OMSs with large pore size and short straight channels are usually required, since the shape and curvature of the pores was found to be important for the diffusion of molecules or ions, which ultimately influenced the loading or grafting density of organic ligands, as well as the adsorption capacity.22,23 In this respect, SBA-15 mesoporous silicas with larger pore size, thicker wall and better stability than the initially reported M41S seem to be more promising.24,25 Moreover, the morphology and internal channel structure of SBA-15 can be readily tuned by using organic reagents, strong electrolytes, or metal ions as additives.26–29 On the other side, extensive efforts are also directed to the design and screening of excellent organic ligands with strong affinity to the target molecules or metal ions, which can impart the OMSs with highly-efficient and selective adsorption ability.30
Palladium, as a precious metal, is of great value in modern industries because of its versatile physical and chemical properties, such as catalysis, electronic and medical devices fabrication, etc.31 However, the limited palladium resource in earth's crust with very low availability cannot fulfill the increasing demand of palladium nowadays.32 It is urged to develop economic and green separation technologies for the effective recovery of palladium from industrial wastes. Recently, recycling of the fission palladium products in spent nuclear fuel has been highlighted because of their considerable amount and benign radioactivity level.33,34 According to a previous report, around 11 kg palladium could be generated in every metric ton of spent fuel of reactors, which would greatly supplement the current shortage of palladium resource.35
Lately, the marriage between ion recognition and nano-materials has opened new perspectives for the construction of adsorbents with specific recognition to metal ions.36–39 We previously reported the development of new silica particles functionalized with macrocyclic ligands like crown ether37 and calixcrown ether38 which exhibited high affinity and selectivity toward palladium ions in HNO3 media. The coordination mechanism between the macrocyclic ligands and Pd(II) could not be well-explained by the conventional hole-size fitting concept in the supramolecular chemistry. More efforts are being made to investigate this issue by using spectroscopic techniques.
Polyazamacrocyclic ligands, such as ‘Cyclen’, ‘Cyclam’ and their derivatives, due to the strong binding ability to transition metal ions, are of significance in the area of magnetic resonance imaging and radioactive drugs production.40 By covalently immobilizing polyazamacrocyclic ligands to the surface of silica gels, Guilard's group developed a series of new silica-based adsorbents for the adsorption of dioxygen41 and transition metal ions.42 Electron spin resonance (ESR) spectroscopy was employed to study the binding mechanisms. Besides, they also reported the use of polyazamacrocyclic ligands bound silica gels for effective separation of U(VI) for decontamination of alpha nuclides in radioactive liquid wastes (RLWs).43 As mentioned above, compared to the pristine silica gel particles with relatively small specific surface area and poor porosity, OMSs possess evident structural advantages for adsorption applications.44 However, to our knowledge, polyazamacrocyclic ligands functionalized OMSs with strong binding ability to precious metal ions have been scarcely reported in the literature.
In this study, we report the synthesis of a new class of short-channel SBA-15 mesoporous silica bearing polyazamacrocyclic ligands, which shows selective binding to palladium ions in HNO3 media based on host–guest interaction. Initially, a mesoporous silica precursor containing a large number of halogen atoms was prepared via the co-condensation method. Then, the polyazamacrocyclic ligands, 1,4,7,10-teraazacyclododecane (Cyclen), were covalently grafted into the mesoporous silica matrix through a nucleophilic substitution reaction. The structure and morphology of the Cyclen functionalized mesoporous silicas were characterized. Adsorption behavior toward Pd(II) in aqueous HNO3 solutions was fully investigated. Especially, the selectivity of the Cyclen functionalized mesoporous silicas to Pd(II) in simulated RLWs against various interfering metal ions was evaluated. Moreover, the specific interaction between the Cyclen ligands and Pd(II) was probed based on high resolution X-ray photoelectron spectroscopy (XPS), and, a suggested mechanism involving the synergistic effect of four cyclic amines was proposed.
Experimental
Chemicals
1,4,7,10-Tetraazacyclododecane (Cyclen) was synthesized according to Stephan's work45 and the structure was confirmed by 1H and 13C NMR spectra. Anhydrous magnesium chloride (MgCl2), Pluronic P123 (average molecular weight ∼ 5800), tetramethoxysilane (TMOS) and chloro(trimethoxy)silane (CPTMS) were supplied by Sigma-Aldrich. Other analytical grade chemicals including hydrochloric acid (HCl), potassium carbonate (K2CO3), potassium iodide (KI) and ethanol were commercially received and used without further purification. Deionized water with resistivity >18 MΩ cm was obtained from a Milli-Q water purification system. Dry tetrahydrofuran (THF) was anhydrously treated in advance with sodium and benzophenone before use.Characterizations
Powder small angle X-ray diffraction (SAXRD) data were collected on a D8 discover with a Cu Kα radiation at room temperature. Fourier transform infrared (FT-IR, 4000–400 cm−1) spectra were performed on Nicolet Nexus 470 in KBr matrix. N2 adsorption–desorption measurements were performed by using Surface Area and Porosity Analyzer (Nava 3200e). Specific surface area was obtained by the Brunauer–Emmett–Teller (BET) method and pore size distribution was calculated by the Discrete-Fourier-Transform (DFT) method. Surface morphology and micro-structure of pore channels were examined by scanning electron microscope (SEM, LEO 1530) and transmission electron microscope (TEM, HT-7700, Japan). Elemental analysis of C, H and N was performed by CE-440 elemental analyzer. Thermogravimetric analysis (TGA) was performed on SDT-Q600 in temperature range 60–1000 °C. X-ray photoelectron spectroscopy (XPS) was carried out on PHI Quantera SXM spectrometer with a monochromatic Al Kα X-ray source (1486.6 eV, 15 mA, 10 kV). The base pressure in the measuring chamber was kept in 10−9 Torr and the binding energy was corrected using the C 1s peak as the reference energy at 284.8 eV. The data was analyzed on the XPSPEAK software. Concentration of metal ions was determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES) and atomic absorption spectroscopy (AAS, HITACHI Z-2000).Synthesis of precursor SBA-15-Cl
Mesoporous silica precursor was prepared through a co-condensation reaction by using chloro(trimethoxy)silane (CPTMS) and tetramethoxysilane (TMOS).16,46 Firstly, Pluronic P123 (5.9 g, 1.0 mmol) and magnesium chloride (8.6 g, 0.12 mmol) were dissolved in an aqueous HCl solution under vigorous stirring. After 7.8 g TMOS was added, the resulting solution was hydrolyzed at 40 °C for 2 h. Then, 1.8 g CPTMS was added dropwise into the solution. The reaction lasted for another 22 h at 40 °C under stirring. The molar ratio of the reactants was 0.85 TMOS/0.15 CPTMS/0.017 P123/1.5 MgCl2/5.9 HCl/194.5 H2O. The obtained mixture was transferred to a Teflon-lined stainless-steel autoclave and then aged at 100 °C for additional 24 h. The as-synthesized silica precursor, denoted as SBA-15-Cl, was collected by filtration and dried at 80 °C under vacuum for 16 h. The template Pluronic P123 was removed by Soxhlet extraction in 500 mL ethanol/HCl (v/v = 494/6) solution for 4 days.Synthesis of Cyclen functionalized mesoporous silica SBA-15-Cyclen
1.0 g SBA-15-Cl precursor and 3.5 g Cyclen were mixed in 150 mL dry THF, followed by the addition of 10.0 g K2CO3 and 4.0 g KI as catalysts. Under nitrogen atmosphere, the mixture was stirred at 80 °C for 48 h. The final product was separated by vacuum filtration and washed with deionized water and ethanol, respectively, until the effluent was in neutral and no excess potassium cations were detected by atomic absorption spectroscopy (AAS). Then, the product was dried at 80 °C under vacuum for 24 h.Pd(II) adsorption experiments
Adsorption toward Pd(II) in aqueous HNO3 solution was investigated by batch operation in plastic tubes. Briefly, 10 mg SBA-15-Cyclen was mixed with Pd(II) solution immersed in a 25 °C constant temperature oscillator. The acidity of the solutions was adjusted to desired values in advance, and the phase rate was controlled to be 0.8 g L−1. After shaken for 48 h, the Pd(II)-loaded SBA-15-Cyclen was separated by centrifuging. The concentration of residual Pd(II) in aqueous phase was measured by AAS. The adsorption efficiency E (%) and adsorption capacity q (mg g−1) were calculated by the following equations.
 | (1) |
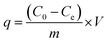 | (2) |
To evaluate the selectivity of the SBA-15-Cyclen to Pd(II), batch experiment was performed in simulated HLLW with a phase rate of 6 g L−1 and a shaking time of 48 h. The simulated RLWs contained a complex mixture of metal ions in nitric acid with each concentration equivalent to that of real RLWs generated during the spent fuels reprocessing of a light water reactor (LWR).37 After reaching the adsorption equilibrium, the residual metal ions in aqueous solution were measured by ICP-AES, except for the Cs(I) detected by AAS.
Results and discussion
Synthesis and characterization of SBA-15-Cyclen
The synthetic route of the Cyclen decorated short-channel SBA-15 mesoporous silica (SBA-15-Cyclen) was illustrated in Fig. 1. Firstly, chlorine modified silica precursor SBA-15-Cl with well-established mesoporous channels and abundant reactive sites was synthesized by a typical co-condensation method. After removing the template Pluronic P123, Cyclen ligands were covalently grafted onto the surface and pore channels of the precursor through a nucleophilic substitution reaction. With the talented polyazamacrocyclic ligands, the SBA-15-Cyclen is expected to show affinity to Pd(II) in aqueous solutions. | ||
| Fig. 1 Synthesis of Cyclen ligands decorated short-channel SBA-15 mesoporous silicas for selective capturing of Pd(II) in HNO3 solution. | ||
The ordered degree of pore channel for the SBA-15-Cl and SBA-15-Cyclen was examined by SAXRD as shown in Fig. 2. The diffraction peaks of (100), (110), and (200) for precursor SBA-15-Cl were indicative of a typical two dimensional hexagonal symmetry (P6mm). After grafting of the Cyclen ligands, the strong diffraction peak of (100) for SBA-15-Cyclen was well-preserved, suggesting that the ordered structure was maintained after the post modification. However, it can be seen that the intensities of the diffraction signals slightly decreased, especially those corresponding to (110) and (200) peaks. The change implied that the ordering degree of the mesoporous silicas was, to some extent, weakened during the post reaction with Cyclen in THF solution. Such phenomenon usually happens for the modification or functionalization of OMSs due to the mismatch between the pristine silicate framework and organic moieties attached to the inner pore channel.47
The SEM and TEM images showed the surface morphology and microstructure of the mesoporous silicas. The SEM images in Fig. 3(a) and (b) show that both the SBA-15-Cl precursors and the Cyclen functionalized mesoporous silicas exhibit a well cylindrical rod-like structure. Compared to normal SBA-15, shorter and uniform channels (0.5–1.0 μm) can be identified, which are not agglomerated in longer secondary particles. Besides, the ordered silicate framework of the precursor SBA-15-Cl with uniform pore size of ca. 8 nm is clearly seen in the TEM image (Fig. 3(c)). The post modification with Cyclen ligands did not damage the cylindrical pore. The obtained SBA-15-Cyclen preserved the mesoporous regularity as the precursor (Fig. 3(d)). This can be attributed to the well physicochemical stability of mesoporous silicas during the post modification.
 | ||
| Fig. 3 SEM images of SBA-15-Cl (a) and SBA-15-Cyclen (b), TEM images of SBA-15-Cl (c) and SBA-15-Cyclen (d). | ||
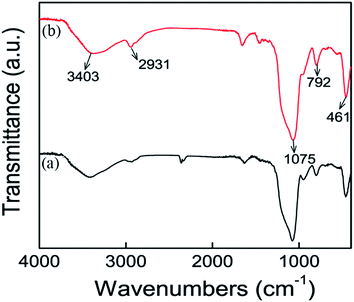
FT-IR spectra of the SBA-15-Cl and SBA-15-Cyclen were obtained for identifying the chemical structure. As shown in Fig. 4(a), the typical vibration peaks located at 3403 cm−1, 2931 cm−1 and 1075 cm−1, correspond to the Si–OH, –CH2– and Si–O–Si in the precursor SBA-15-Cl, respectively. The grafting of Cyclen ligands resulted in the enhanced intensity of the Si–OH and –CH2– vibrations, suggesting the expected anchoring of the polyazamacrocyclic ligands. It is noteworthy that the characteristic vibration corresponding to the –NH– units in the Cyclen ligands was overlapped by the broad Si–OH absorption band, hardly differentiated in the spectra.
Besides, elemental analysis of C, H, and N was performed to quantify the loading amount of Cyclen ligands to the SBA-15 mesoporous silicas. The data summarized in Table 1 show that the precursor SBA-15-Cl contains 10.72 wt% C and 3.23 wt% H, respectively. With the introduction of Cyclen, the SBA-15-Cyclen shows evidently increased amount of C (18.63 wt%), H (4.25 wt%), and 5.41 wt% N. Based upon the amount of N, it can be estimated that approximately 16.7 wt% of Cyclen ligands (∼0.97 mmol g−1) were grafted to the mesoporous silicas. Such a high loading amount of functional moieties is attributed to the structural advantage of the substrate materials with short-channel and large pore size, which facilitated the diffusion of the molecules in reaction mixture.22,23
| Samples | Elemental analysis | ||
|---|---|---|---|
| C wt% | H wt% | N wt% | |
| SBA-15-Cl | 10.72 | 3.23 | Null |
| SBA-15-Cyclen | 18.63 | 4.25 | 5.41 |
N2 adsorption–desorption isotherm measurement and pore-size analysis were performed to inspect surface and pore property of the mesoporous silicas. Both curves in Fig. 5(a) showed typical type-IV isotherms with clear H1-type broad hysteresis loops in the partial pressure range from 0.40 to 0.65, indicating the existence of uniform mesopores for SBA-15-Cl and SBA-15-Cyclen. The parameters of pore structure and specific surface area of the samples were calculated and summarized in Table 2. The precursor SBA-15-Cl had a BET specific surface area of 399.7 m2 g−1 with pore volume of 0.36 cm3 g−1 and pore size of 3.79 nm. The statistical pore size with a smaller value than that visualized in the TEM image suggested there existed substantial micropores in the silica matrix, which was consistent with the pore size distribution curve (Fig. 5(b)). After grafting with Cyclen ligands, SBA-15-Cyclen showed decreased specific surface area of 120.5 m2 g−1 with pore volume of 0.14 cm3 g−1 and pore size of 3.46 nm. This normally happens for the organic functionalization of OMSs, which should be attributed to partial blocking of the pore channels by the introduced organic species.
 | ||
| Fig. 5 N2 adsorption–desorption isotherms (a) and pore size distributions curves (b) of SBA-15-Cl and SBA-15-Cyclen. | ||
| Sample | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) |
|---|---|---|---|
| SBA-15-Cl | 399.7 | 0.36 | 3.79 |
| SBA-15-Cyclen | 120.5 | 0.14 | 3.46 |
Adsorption behavior study
The adsorption rate is an important factor for evaluating the performance of an adsorbent while providing insights into the pathway of adsorption process. The effect of contact time on the adsorption of Pd(II) by the functionalized mesoporous silicas was investigated in 1.0 mol L−1 HNO3 solution containing 50 mg L−1 Pd(II). The curve in Fig. 6(a) shows that the uptake of Pd(II) onto the SBA-15-Cyclen was relatively fast in the first 12 h, with 90% Pd(II) removed from the HNO3 solution. Then, the adsorption gradually reached equilibrium after 24 h. It should be pointed out that the adsorption kinetics of the SBA-15-Cyclen is not very fast compared to some of the reported mesoporous silica adsorbents.37,38 Considering the structural characteristics of the mesoporous silica matrix, namely, short and straight channels and large pore size which should have promoted the mass transfer, it could be inferred that the supramolecular coordination between the anchored Cyclen ligands and the Pd(II) ions in HNO3 solution was the rate-controlling step, which might involve the conformational change and/or coordination bonds generation, leading to a relatively slowly adsorption kinetics. | ||
| Fig. 6 (a) Adsorption of Pd(II) by SBA-15-Cyclen as a function of time, (b) the fitted curve based on the pseudo-second-order model: [Pd(II)] = 50 mg L−1, T = 298 K, [m/v] = 0.8 g L−1. | ||
To better understand the underlying kinetics mechanism, the experiment data was fitted by using pseudo-first-order kinetic model48 and pseudo-second-order kinetic model.49 The equations of the kinetic models are presented as follows:
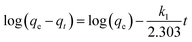 | (3) |
 | (4) |
| Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|
| k1 (h−1) | R2 | qt,cal (mg g−1) | k2 (g mg−1 h−1) | R2 | qt,cal (mg g−1) |
| 0.1347 | 0.976 | 37.7 | 0.0043 | 0.999 | 49.4 |
Influence of the initial Pd(II) concentration in 1.0 mol L−1 HNO3 solution on the adsorption capacity of SBA-15-Cyclen was examined by batch operation in a thermostat. It can be seen in Fig. 7 that, with raising the Pd(II) concentration, the adsorption capacity of SBA-15-Cyclen increases rapidly at low concentration (less than 100 mg L−1), followed by a slow-down to equilibrium due to the limited number of the available adsorption sites.
 | ||
| Fig. 7 Data fitting by using Langmuir and Freundlich isotherm models for adsorption of Pd(II) by SBA-15-Cyclen, T = 298 K, contact time = 32 h, [m/v] = 0.8 g L−1. | ||
To explore the adsorption mechanism, the Langmuir and Freundlich adsorption isotherm models were employed to fit the experimental data. The Langmuir model is common regarded as a dynamic single-layer sorption process with equal energy of adsorption, which can be expressed as:
 | (5) |
 | (6) |
| Langmuir model | Freundlich model | ||||
|---|---|---|---|---|---|
| qmax (mg g−1) | KL (L mg−1) | R2 | 1/n | KF ((mg g−1) (L mg)1/n) | R2 |
| 76.9 | 0.023 | 0.997 | 2.43 | 49.4 | 0.837 |
According to previous reports,51,52 palladium in HNO3 solution mostly exists by the form of metal-nitrate complex, such as ([Pd(NO3)n]2−n, n = 1–4). Hence, HNO3 concentration is an important factor affecting the binding of Cyclen ligands to the Pd(II) ions. Fig. 8 shows the effect of HNO3 concentration (0.1–5.0 mol L−1) on the adsorption rate of Pd(II) by the mesoporous silicas. Obviously, the precursor SBA-15-Cl has no affinity to Pd(II) under any condition. Due to the binding ability of the Cyclen ligands, SBA-15-Cyclen shows effective adsorption toward Pd(II) under a wide range of HNO3 concentration. Meanwhile, it is noted that the adsorption rate of Pd(II) decreases with increasing the HNO3 concentration. This should be explained by the protonation of the cyclic N atoms in the Cyclen ligands and the competing effect of the excess nitrate groups.
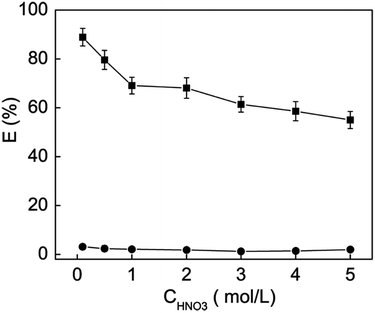 | ||
| Fig. 8 Influence of HNO3 concentration on the Pd(II) adsorption rate by SBA-15-Cyclen and SBA-15-Cl: T = 298 K, contact time = 32 h, [m/v] = 0.8 g L−1, [Pd(II)] = 50 mg L−1. | ||
Besides, to understand the thermodynamic properties of the adsorption process, the effect of temperature on the adsorption of Pd(II) by SBA-15-Cyclen was studied. The adsorption capacity at different operational temperature varying from 285 K to 305 K are displayed in Fig. 9(a). Apparently, with the rise of temperature, the adsorption capacity of Pd(II) gradually increased, indicating that adsorption ability of the SBA-15-Cyclen was enhanced at higher temperature.
 | ||
Fig. 9 Effect of temperature on the Pd(II) adsorption by SBA-15-Cyclen: [Pd(II)] = 50 mg L−1, T = 298 K, [m/v] = 0.8 g L−1 (a); linear plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd vs. 1/T for the adsorption of Pd(II) (b). Kd vs. 1/T for the adsorption of Pd(II) (b). | ||
The thermodynamic parameters (ΔH0, ΔG0, ΔS0) can be calculated from the following Van't Hoff equation:53
 | (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd vs. 1/T (Fig. 9(b)). The value of the Gibbs free energy change ΔG0 (kJ mol−1) was calculated by the following equations:
Kd vs. 1/T (Fig. 9(b)). The value of the Gibbs free energy change ΔG0 (kJ mol−1) was calculated by the following equations:| ΔG0 = ΔH0 − TΔS0 | (8) |
The obtained thermodynamic parameters are listed in Table 5. The positive value of ΔH0 (9.77 kJ mol−1) reveals the endothermic nature of adsorption process. It might be explained that Pd(II) ions by the form of ([Pd(NO3)n]2−n, n = 1–4) in HNO3 media have to be stripped NO3− groups to release the empty electron orbitals before the coordination to the Cyclen ligands, and the endothermicity of the dissociation processes suppresses the exothermicity of Pd(II) transfer to the surface of adsorbent.54 The positive value of ΔS0 (85.98 J (mol−1 K−1)) suggests the increased randomness at the solid/solution interface during the adsorption of Pd(II) on SBA-15-Cyclen, while implying some changes in the structure of adsorbent after binding to Pd(II). Moreover, the negative value of ΔG0 shows that the adsorption process is spontaneous. The ΔG0 value become further negative with rising the temperature, suggesting the adsorption toward Pd(II) by the SBA-15-Cyclen would more easily happen at high temperature.
| T (K) | ΔH0 (kJ mol−1) | ΔS0 J (mol−1 K−1) | ΔG0 (kJ mol−1) |
|---|---|---|---|
| 285 | 9.77 | 85.98 | −14.73 |
| 295 | −15.59 | ||
| 305 | −16.45 |
Selectivity and reusability
The selectivity of the SBA-15-Cyclen to Pd(II) against interfering metal ions was evaluated. The test solution to simulate radioactive liquid wastes (RLWs) was previously prepared, which contained Pd(II) and other 13 kinds of metal ions including Na(I), K(I), Cs(I), Sr(II), Ba(II), Cd(II), Ni(II), Nd(III), Cr(III), Ru(III), Fe(III), Zr(IV) and Mo(VI) in 1.0 mol L−1 HNO3.37 Batch adsorption was implemented with a phase rate of 5.0 g L−1. The concentrations of the metal ions in simulated RLWs as well as the adsorption rate E (%) by SBA-15-Cyclen and SBA-15-Cl were summarized in Table 6. Evidently, the SBA-15-Cyclen showed good selectivity to Pd(II) in the presence of the above-mentioned interferences with a satisfied adsorption rate of 90.2%. In comparison, the precursor SBA-15-Cl, due to the absence of the Cyclen ligands, showed quite poor affinity to Pd(II) as well as other metal ions. This could be reasonably explained by the specific coordination between the Cyclen ligands and the Pd(II) ions in HNO3 solutions. While the co-existing lanthanides ions, transition metal ions, alkali and alkaline earth metal ions could not form stable complexes with the Cyclen ligands either due to the limited coordination sites or the steric effect.| Element | Original amount (g L−1) | Adsorption rate E (%) | |
|---|---|---|---|
| SBA-15-Cl | SBA-15-Cyclen | ||
| K | 0.340 | 2.9 ± 0.4 | 4.1 ± 0.2 |
| Na | 1.139 | 1.3 ± 0.6 | 2.5 ± 0.4 |
| Cs | 0.344 | 4.1 ± 0.3 | 5.3 ± 0.2 |
| Sr | 0.169 | 1.1 ± 0.5 | 0.1 ± 0.1 |
| Ba | 0.448 | 0.2 ± 0.2 | 1.4 ± 0.3 |
| Cd | 0.045 | 2.7 ± 0.8 | 1.8 ± 0.8 |
| Ni | 0.055 | 1.8 ± 0.7 | 2.2 ± 0.6 |
| Nd | 0.245 | 0.4 ± 0.1 | 0.3 ± 0.2 |
| Cr | 0.067 | 1.2 ± 0.2 | 1.5 ± 0.4 |
| Ru | 0.074 | 2.0 ± 0.3 | 3.9 ± 0.7 |
| Fe | 0.080 | 2.6 ± 0.6 | 3.4 ± 1.2 |
| Mo | 0.695 | 4.2 ± 0.5 | 9.1 ± 1.4 |
| Zr | 0.135 | 4.5 ± 0.2 | 7.5 ± 1.1 |
| Pd | 0.337 | 0.2 ± 0.1 | 90.2 ± 1.6 |
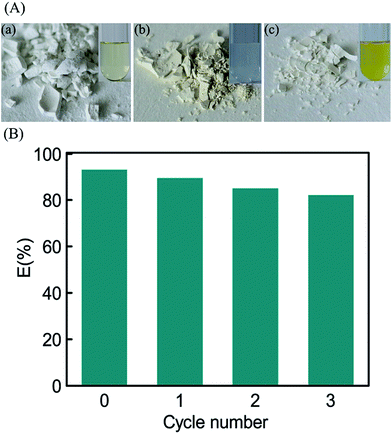
Effective stripping of loaded Pd(II) is of great value for the recovery of the precious metal and regeneration of the SBA-15-Cyclen. It has been reported that thiourea, with soft S donor, can efficiently strip the Pd(II) off the matrix in acid media by forming a stable positively-charged complex.55 Hence, 1% thiourea in 0.5 mol L−1 HNO3 was utilized for elution of the Pd(II)-loaded SBA-15-Cyclen. Fig. 10(A) records the visual color change of the test solutions and the adsorbents in an adsorption–elution operation (see ESI for details†), indicating an effective stripping of the adsorbed Pd(II) ions from the SBA-15-Cyclen. Besides, reusability of the mesoporous silicas was evaluated. In a 4-cycle experiment, the SBA-15-Cyclen only showed 10% decrease in the adsorption rate of Pd(II) (Fig. 10(B)). The slight decrease in adsorption efficiency could be attributed to incomplete stripping of the adsorbed Pd(II) ions during the elution process, leading to the loss of a few effective adsorption sites on the surface of the SBA-15-Cyclen.54 Overall, the adsorbed Pd(II) by the Cyclen functionalized mesoporous silicas can be readily recovered, and the regenerated adsorbents possess the potential for cycle use.
Coordination mechanism discussion
The mechanism for the selective binding to noble metal ions by polyazamacrocyclic ligands in nitric acid solutions is still undetermined at present. People have reported that cyclic thioethers with different ring size could form mono-, bi-, and poly-nuclear complexes with platinum metals due to the great affinity of S donors and suitable size-match.56 In this study, it is believed that the cyclic N donors in the Cyclen ligands should be responsible for the binding to Pd(II). To better understand the underlying mechanism, high resolution XPS analysis was performed to identify the involved interactions between the Cyclen ligands anchored to the mesoporous silica matrix and Pd(II) in HNO3 solutions.Fig. 11(a) shows the overall XPS surveys of the SBA-15-Cl, SBA-15-Cyclen and its counterpart after the binding to Pd(II), labelled as SBA-15-Cyclen-Pd. Firstly, these XPS spectra provide more evidences for the determination of chemical structures of the functionalized mesoporous silicas. For instance, the typical Cl 2p signal present at 200.2 eV in the spectrum of SBA-15-Cl proves the incorporation of chlorine atoms into the silica matrix. For the SBA-15-Cyclen, due to the introduction of the Cyclen ligands which replaced the chlorine atoms, the Cl 2p signal disappears whereas a new distinct N 1s signal shows up at 399.4 eV. In addition, the adsorbed-Pd on the SBA-15-Cyclen is evidenced by the presence of Pd 3d signal (Fig. 11(b)), which exhibits two asymmetrical peaks assigned to Pd 3d5/2 (337.4 eV) and Pd 3d3/2 (342.8 eV) with a spin–orbit splitting of ∼5.4 eV.57
Furthermore, through the deconvolution analysis of high resolution XPS spectra, more information concerning the specific chemical species or functional groups can be obtained, which may help to shed light on the interactions or reactions happened on the solid surfaces. As shown in Fig. 11(c), the deconvolution of the C 1s spectra of SBA-15-Cyclen resulted in three components: C–H (284.0 eV), C–C (284.8 eV) and C–N (285.7 eV). The appearance of the C–N signal was certainly attributed to the anchoring of the Cyclen ligands with cyclic amine groups. After Pd(II) adsorption, the C 1s spectra of SBA-15-Cyclen-Pd by the same deconvolution processing was observed in Fig. 11(d). By contrast, the peaks of C–H (284.0 eV) and C–C (284.8 eV) kept almost unchanged. Nevertheless, the binding energy of the C–N peak was shifted to the lower binding energy (285.2 eV), implying that the C–N groups in the Cyclen ligands were involved in the coordination with Pd(II).58
The deconvolution analysis of N 1s spectra was implemented to further examine the coordination sites of the Cyclen ligands. The N 1s high resolution spectra of SBA-15-Cyclen was composed of two peaks with binding energy of 399.1 eV and 398.4 eV with the intensity ratio of 2.1 (Fig. 11(e)). The peak at higher binding energy 399.1 eV was assigned to cyclic free secondary amines (–NH–) in Cyclen molecules, and the other peak at 398.4 eV was originated from cyclic specific amines connecting to the mesoporous silica surface (–N(R)–).59,60 After the binding to Pd(II), both the N 1s component signals shifted to higher binding energy, i.e., 399.4 eV for the –NH– species and 398.6 eV for the –N(R)– species, suggesting that the four cyclic amines synergistically participated in the supramolecular coordination with Pd(II) (Fig. 11(f)). These results were in accordance with the above analysis of C–N signals in C 1s spectra. The increased binding energy was due to the formation of N–Pd(II) coordination bonds, where the cyclic amines of Cyclen hosts shared the electron pair with the Pd(II) guests and thus the electron density of N atoms was reduced.61 Besides, it is worth noting that a new N 1s peak of SBA-15-Cyclen after the adsorption of Pd(II) appeared at 405.5 eV representative of NO3− groups.60 This implied that the NO3− groups, initially located in the inner coordination orbits of Pd(II) as [Pd(NO3)n]2−n (n = 1–4) in HNO3 solutions, were replaced by the Cyclen ligands and transferred to outer orbits as negative-ions to keep the charge balance of Pd-complex structure.
Based on the above XPS analysis, it could be inferred that, similar to the coordination manner of cyclic thioethers, all the –NH– and –N(R)– units in the Cyclen ligands synergistically interacted with Pd(II) in HNO3 solutions to form a stable tetra-coordinate complex. Meanwhile, the NO3− groups in the [Pd(NO3)n]2−n complexes were replaced and transferred to the periphery as charge neutralizers. The detailed mechanism could be expressed as follows:
| [HmLs]sm+ + [Pd(NO3)n]aq2−n = m[H]aq+ + [(PdLs)(NO3)n]s2−n |
Conclusions
In summary, a novel class of short-channel SBA-15 mesoporous silica functionalized with polyazamacrocyclic ligands was synthesized for selective capturing of palladium ions in HNO3 solutions. A two-step synthesis protocol was established involving the co-condensation synthesis of a chlorine incorporated mesoporous silica precursor, followed by the covalent grafting of Cyclen ligands via nucleophilic substitution reaction. The functionalized mesoporous silica SBA-15-Cyclen exhibited large specific surface area, short length channel, large pore size and high grafting density of the Cyclen ligands. Due to the specific binding ability of the Cyclen ligands, the SBA-15-Cyclen showed good adsorption capacity and high selectivity to Pd(II) in HNO3 solutions. Batch experiments revealed that the adsorption of Pd(II) by SBA-15-Cyclen might obey the pseudo-second-order kinetics, and could be interpreted by the Langmuir isotherm model. Thermodynamic data revealed that the adsorption process was spontaneous and endothermic. Moreover, the coordination mechanism between the Cyclen ligands and Pd(II) was investigated based on high resolution XPS survey. A suggested mechanism involving the synergistic effect of four cyclic amines in the Cyclen ligands for the binding of Pd(II) was proposed. This kind of polyazamacrocyclic ligands functionalized SBA-15 mesoporous silicas might be promising for selective enrichment of palladium as well as other precious metals in acidic media.Acknowledgements
This work was supported by Program for Changjiang Scholars and Innovative Research Team in University (IRT13026) and National Natural Science Foundation of China under Projects 51425403, 51473087 and U1430234.Notes and references
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. Chu, D. H. Olson, E. W. Sheppard, S. B. Mccullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834 CrossRef CAS.
- A. Stein, B. J. Melde and R. C. Schroden, Adv. Mater., 2000, 12, 1403 CrossRef CAS.
- F. Hoffmann, M. Cornelius, J. Morell and M. Froba, Angew. Chem., Int. Ed., 2006, 45, 3216 CrossRef CAS PubMed.
- F. Hoffmann and M. Froba, Chem. Soc. Rev., 2011, 40, 608 RSC.
- Z. Wu and D. Zhao, Chem. Commun., 2011, 47, 3332 RSC.
- A. Walcarius and L. Mercier, J. Mater. Chem., 2010, 20, 4478 RSC.
- W. G. Lin, F. Wei, Q. Hou, T. Y. Zhang, Y. K. Wang and J. H. Zhu, Microporous Mesoporous Mater., 2012, 156, 233 CrossRef CAS.
- Z. X. Li, J. C. Barnes, A. Bosoy, J. F. Stoddart and J. I. Zink, Chem. Soc. Rev., 2012, 41, 2590 RSC.
- P. P. Yang, S. L. Gai and J. Lin, Chem. Soc. Rev., 2012, 41, 3679 RSC.
- D. Pérez-Quintanilla, I. Del Hierro, M. Fajardo and I. Sierra, Microporous Mesoporous Mater., 2006, 89, 58 CrossRef.
- J. Huang, M. Ye, Y. Qu, L. Chu, R. Chen, Q. He and D. Xu, J. Colloid Interface Sci., 2012, 385, 137 CrossRef CAS PubMed.
- A. Shahbazi, H. Younesi and A. Badiei, Chem. Eng. J., 2011, 168, 505 CrossRef CAS.
- Y. Song, Y. Du, D. Lv, G. Ye and J. Wang, J. Hazard. Mater., 2014, 274, 221 CrossRef CAS PubMed.
- W. Zhang, X. He, G. Ye, R. Yi and J. Chen, Environ. Sci. Technol., 2014, 48, 6874 CrossRef CAS PubMed.
- Y. Leng, G. Ye, J. Xu, J. Wei, J. Wang and J. Chen, J. Sol-Gel Sci. Technol., 2013, 66, 413 CrossRef CAS.
- P. Makowski, X. Deschanels, A. Grandjean, D. Meyer, G. Toquer and F. Goettmann, New J. Chem., 2012, 36, 531 RSC.
- D. D. Asouhidou, K. S. Triantafyllidis, N. K. Lazaridis and K. A. Matis, Colloids Surf., A, 2009, 346, 83 CrossRef CAS.
- T. Kang, Y. Park, K. Choi, J. S. Lee and J. Yi, J. Mater. Chem., 2004, 14, 1043 RSC.
- I. I. Slowing, J. L. Vivero-Escoto, B. G. Trewyn and V. S. Y. Lin, J. Mater. Chem., 2010, 20, 7924 RSC.
- Z. L. Yang, Y. F. Lu and Z. Z. Yang, Chem. Commun., 2009, 2270 RSC.
- A. H. Janssen, P. Van Der Voort, A. J. Koster and K. P. de Jong, Chem. Commun., 2002, 1632 RSC.
- J. Fan, J. Lei, L. Wang, C. Yu, B. Tu and D. Zhao, Chem. Commun., 2003, 2140 RSC.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS PubMed.
- V. Meynen, P. Cool and E. F. Vansant, Microporous Mesoporous Mater., 2009, 125, 170 CrossRef CAS.
- L. Wang, T. Qi, Y. Zhang and J. Chu, Microporous Mesoporous Mater., 2006, 91, 156 CrossRef CAS.
- S. Kubo and K. Kosuge, Langmuir, 2007, 23, 11761 CrossRef CAS PubMed.
- X. Wang, J. C. C. Chan, Y. Tseng and S. Cheng, Microporous Mesoporous Mater., 2006, 95, 57 CrossRef CAS.
- W. Guo, J. Park, M. Oh, H. Jeong, W. Cho, I. Kim and C. Ha, Chem. Mater., 2003, 15, 2295 CrossRef CAS.
- K. Ariga, A. Vinu, J. P. Hill and T. Mori, Coord. Chem. Rev., 2007, 251, 2562 CrossRef CAS.
- R. Ruhela, A. K. Singh, B. S. Tomar and R. C. Hubli, RSC Adv., 2014, 4, 24344 RSC.
- C. R. M. Rao and G. S. Reddi, TrAC, Trends Anal. Chem., 2000, 19, 565 CrossRef CAS.
- Z. Kolarik and E. V. Renard, Platinum Met. Rev., 2003, 47, 123 CAS.
- Z. Kolarik and E. V. Renard, Platinum Met. Rev., 2003, 47, 74 CAS.
- Z. Kolarik and E. V. Renard, Platinum Met. Rev., 2005, 49, 79 CrossRef CAS.
- R. Yi, G. Ye, D. Pan, F. Wu, M. Wen and J. Chen, J. Mater. Chem. A, 2014, 2, 6840 CAS.
- F. Bai, G. Ye, G. Chen, J. Wei, J. Wang and J. Chen, Sep. Purif. Technol., 2013, 106, 38 CrossRef CAS.
- Y. Leng, J. Xu, J. Wei and G. Ye, Chem. Eng. J., 2013, 232, 319 CrossRef CAS.
- A. Zaghbani, R. Tayeb, M. Dhahbi, M. Hidalgo, F. Vocanson, I. Bonnamour, P. Seta and C. Fontàs, Sep. Purif. Technol., 2007, 57, 374 CrossRef CAS.
- R. Delgado, V. Felix, L. M. P. Lima and D. W. Price, Dalton Trans., 2007, 2734 RSC.
- G. Dubois, R. Tripier, S. Brandès, F. Denat and R. Guilard, J. Mater. Chem., 2002, 12, 2255 RSC.
- C. Gros, F. Rabiet, F. Denat, S. Brandes, H. Chollet and R. Guilard, J. Chem. Soc., Dalton Trans., 1996, 1209 RSC.
- F. Barbette, F. Rascalou, H. Chollet, J. L. Babouhot, F. Denat and R. Guilard, Anal. Chim. Acta, 2004, 502, 179 CrossRef CAS.
- B. Yan and Q. Li, Microporous Mesoporous Mater., 2014, 196, 284 CrossRef CAS.
- S. W. Kohl, K. Kuse, M. Hummer, H. Schumann, C. Muegge, K. Janek and H. Weisshoff, Z. Naturforsch., B: J. Chem. Sci., 2007, 62, 397 CAS.
- C. Z. Yu, J. Fan, B. Z. Tian, D. Y. Zhao and G. D. Stucky, Adv. Mater., 2002, 14, 1742 CrossRef CAS.
- S. Gago, J. A. Fernandes, J. P. Rainho, R. A. Sá Ferreira, M. Pillinger, A. A. Valente, T. M. Santos, L. D. Carlos, P. J. A. Ribeiro-Claro and I. S. Gonçalves, Chem. Mater., 2005, 17, 5077 CrossRef CAS.
- Y. S. Ho and G. McKay, Process Saf. Environ. Prot., 1998, 76, 332 CrossRef CAS.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451 CrossRef CAS.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361 CrossRef CAS.
- C. K. Jorgensen and V. Parthasarathy, Acta Chim. Sin., 1978, 32, 957–965 Search PubMed.
- T. Fujii, S. Egusa, A. Uehara, A. Kirishima, I. Yamagishi, Y. Morita and H. Yamana, J. Radioanal. Nucl. Chem., 2011, 290, 475 CrossRef CAS.
- J. K. Gao, L. A. Hou, G. H. Zhang and P. Gu, J. Hazard. Mater., 2015, 286, 325–333 CrossRef CAS PubMed.
- Y. G. Zhao, J. X. Li, L. P. Zhao, S. W. Zhang, Y. S. Huang, X. L. Wu and X. K. Wang, Chem. Eng. J., 2014, 235, 275–283 CrossRef CAS.
- R. K. Sharma, A. Pandey, S. Gulati and A. Adholeya, J. Hazard. Mater., 2012, 209–210, 285 CrossRef CAS PubMed.
- M. N. Bell, A. J. Blake, R. O. Gould, A. J. Holder, T. I. Hyde, A. J. Lavery, G. Reid and M. Schroder, J. Inclusion Phenom. Macrocyclic Chem., 1987, 5, 169–172 CrossRef.
- I. G. Casella, J. Electrochem. Soc., 2008, 155, D723 CrossRef CAS.
- Z. Zhao, J. Li, T. Wen, C. Shen, X. Wang and A. Xu, Colloids Surf., A, 2015, 482, 258 CrossRef CAS.
- S. Deng and Y. Ting, Water Res., 2005, 39, 2167 CrossRef CAS PubMed.
- J. S. Stevens, A. C. de Luca, M. Pelendritis, G. Terenghi, S. Downes and S. L. M. Schroeder, Surf. Interface Anal., 2013, 45, 1238 CrossRef CAS.
- M. Toupin and D. Bélanger, Langmuir, 2008, 24, 1910 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Sample preparation for XPS survey and the details for reusability evaluation. See DOI: 10.1039/c6ra11778c |
| This journal is © The Royal Society of Chemistry 2016 |