Determination of p-aminohippuric acid with β-cyclodextrin sensitized fluorescence spectrometry†
Abstract
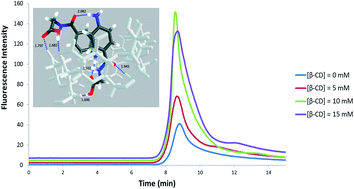
Sensitive spectrofluorometric and liquid chromatography with fluorescence detection methods have been developed for detection and determination of para-aminohippuric acid (PAH), one of the commonly used markers for estimating effective renal plasma flow, in the presence of β-cyclodextrin (β-CD). Fluorescence signals have been enhanced with the addition of β-CD to the drug aqueous solution. The 1 : 2 (host–guest) inclusion between PAH and cyclodextrin was evident by mass spectrometry and density functional theory (DFT) calculations supporting the formation of an excimer state at 355 nm. Factors that affect the PAH interaction with cyclodextrin have been investigated such as the size of the cyclodextrin cavity, the concentration of PAH, the concentration of cyclodextrin and pH effects. A calibration curve was established for the spectrofluorometric data of PAH with β-CD in the concentration range of 0.05–100 μM of PAH and the detection limit was 0.015 μM. HPLC with fluorescence detection was investigated in the presence of β-CD in the mobile phase. It was found that the calibration curve slope increased as the concentration of β-CD increased. Finally urine samples were spiked with 100 μM and 500 μM of PAH and showed recoveries in the range of 104–118% and 99.2–103%, respectively.


 Please wait while we load your content...
Please wait while we load your content...