DOTMA-based amides (DOTMAMs) as a platform for the development of PARACEST MRI contrast agents†
Abstract
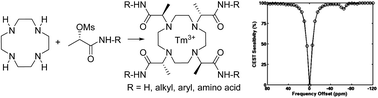
A synthetic methodology leading to previously unknown DOTMA-based secondary amides (DOTMAMs) has been developed. Alkylation of cyclen with L-lactic acid-derived pseudohalides was used as a key step affording the alkyl- and aryl-decorated DOTMAMs. Amino acid-decorated DOTMAMs were obtained via peptide coupling between DOTMA and protected amino acids. Metallation of the DOTMAMs ligand with Tm3+ gave complexes exhibiting proximal amide proton based paramagnetic CEST effects at <−50 ppm relative to water.

 Please wait while we load your content...
Please wait while we load your content...